Page 260 - Polymer-based Nanocomposites for Energy and Environmental Applications
P. 260
232 Polymer-based Nanocomposites for Energy and Environmental Applications
5 5
4 4
Weight % storage 3 2 Weight % storage 3 2
1 1
0 0
0 500 1000 1500 2000 2500 3000 0 500 1000 1500 2000 2500 3000
(A) Surface area [m g ] (B) Surface area [m g ]
2
−1
2
−1
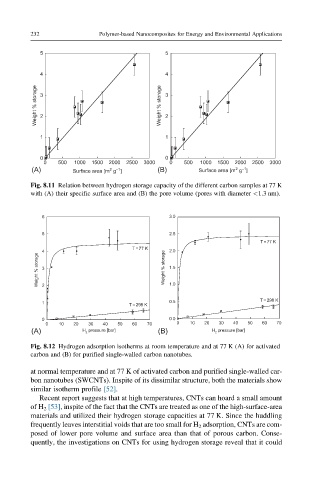
Fig. 8.11 Relation between hydrogen storage capacity of the different carbon samples at 77 K
with (A) their specific surface area and (B) the pore volume (pores with diameter <1.3 nm).
6 3.0
5 2.5
T=77 K
T =77 K
4 2.0
Weight % storage 3 2 Weight % storage 1.5
1.0
1 T =298 K 0.5 T=298 K
0 0.0
0 10 20 30 40 50 60 70 0 10 20 30 40 50 60 70
(A) H 2 pressure [bar] (B) H 2 pressure [bar]
Fig. 8.12 Hydrogen adsorption isotherms at room temperature and at 77 K (A) for activated
carbon and (B) for purified single-walled carbon nanotubes.
at normal temperature and at 77 K of activated carbon and purified single-walled car-
bon nanotubes (SWCNTs). Inspite of its dissimilar structure, both the materials show
similar isotherm profile [52].
Recent report suggests that at high temperatures, CNTs can hoard a small amount
of H 2 [53], inspite of the fact that the CNTs are treated as one of the high-surface-area
materials and utilized their hydrogen storage capacities at 77 K. Since the huddling
frequently leaves interstitial voids that are too small for H 2 adsorption, CNTs are com-
posed of lower pore volume and surface area than that of porous carbon. Conse-
quently, the investigations on CNTs for using hydrogen storage reveal that it could