Page 266 - Pressure Swing Adsorption
P. 266
242 PRESSURE SWING ADSORPTION PSA PROCESSES
243
Table 6.3. Composition of Typical Coke Oven Gas
"'OSie gos
Concentration
Components (% by vol.)
H, 59.99
adsorption pressure
CH, 22.76 i.i - 1.J bar
N, 6.68 co,
co 5.45
C 2 H,1 1.66 feed
o, J.40 gas
co, 1.26
C 2 H{, 0.53 blower
c_,H,, 0.11
C2H2 0.09
0.02
C 1 H 8
final desorption
C 4 H1, 0.02 pressure:
C<iHo 0.02 approx. 50 mbor
C4Ho 0.01
C 4 H 10 0.01
CsH12 0.01 vacuum
pump
Ct,H14 0.01 product gos
C 7 Hu, 0.01
C7Hs 0.01 co,
(>997. by vol.)
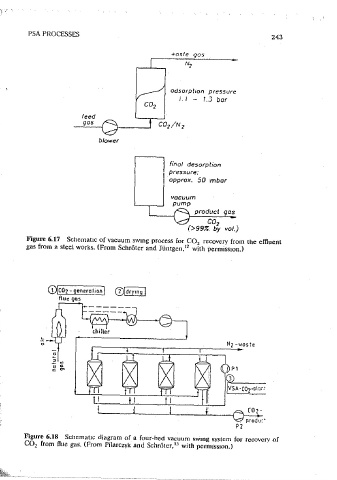
Source:' Ref. 30. Figure 6.17 Schematic of vacuum swmg process for CO recovery from the effluent
2
gas from a steel works. (From Schr5ter and Jiimgen, with permission.)
12
6.5!3 Bergbau - Forschung Process
As an alternative to the zeolite-based hydrogen recovery orocesses developed
by Union Carbide, Bergbau-Forschung has developed eau1vaient processes (i)!co2-9enerationl (D~
using a wide~oore carbon molecular sieve as the adsorbent. A four-bed
flue gas
system is used with five snialler preadsorption beds contaming activated
carbon and Ooerat10g between atm and 1 atm. The process seQuence, which
is basically similar to that used m the four-bed Union Carbide system. is
shown m Figure 6.16. This system has been used orimarily to recover
hydrogen from coke oven gas containing about 60% hydrogen (see Table 6.3).
Hydrogen oroduct purittes as high as 99.999% at a recovery of 85% are
3
claimed, and the largest units have a product rate of 10 4 N m /h. The
oerformance thus appears to be broadly similar to that of the Union Carbide
poiybed system, b1Jt smce only four beds are used, there should be a cap1tai
cost advantage.
6.6 Recovery of CO
2 ~---'~--'~---'i'----~
\dp~~W·
PZ
Carbon dioxide 1s present at relatively high concentrations (15-35%) in the
flue gases from many mdustnes such as steel and lime oroctuction. Since CO 2 Figure 6.18 Scilemat1c diagram of a four~hed vacuum i;Wmg system for recoverv of
ts strongly adsorbed on many adsorbents, mcluding both zeolites and carbon CO2 from flue gas. (From Pilarczyk and Schrt'iter, u with permission.) -