Page 331 - Pressure Swing Adsorption
P. 331
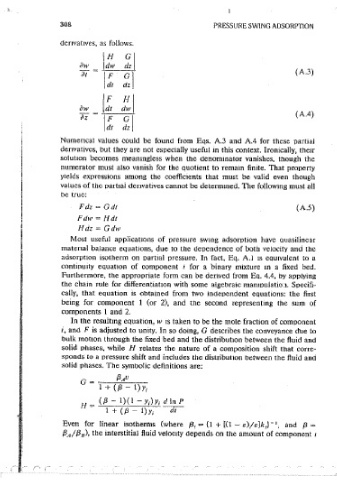
308 PRESSURE SWING ADSORPTION
APPENDIX A
309
denvatrves, as follows.
present, which 1moiies that 1t mav be a ma1or constituent and that the
\H ~I adsorbent may have substantial caoac1tv. In these equations. pressure drop m
aw ldw the adsorbent bed 1s assumed to be negligihle. The resulting equalities.
ill IF GI (A.3) corresponding to the first two of Eq. A.5 arc:
dt dz. I
{3 AIJ
/F !.I dz - ~-'-"--- dt
- I + ( {3 - I) y,
aw ldt I
Tz' (A.4) t (A.6)
I :i Yzl i dy. = (/3 - 1)(1 - Y,)Y, d In P
Numencal values could be found from Eqs. A.3 and A.4 for these partial ' 1+({3-l)y, dt
i
denvativcs, but they are not especially useful m this context. Ironically, thetr
soiutmn becomes meaningless when the denominator vanishes, though the These are eomvaJent to Eus. 4.7 and 4.8, and they are called charactertstlc
numerator must also vanish for the quotient to remam finite. That property eauat10ns. The former defines characteristic traJectories aiong the hed axts
yields expressions among the coefficients that must be valid even though j with respect to time. The latter. which must be soived stmultaneously with
values of the oarttai ctenvatives cannot be detenmned. The following must all the former, defines the composition vanation along each traJectorv (e.g., as
be true: pressure varies). Although characteristics may appear to be arbitrary lines or
curves, they are not. At each position and time there 1s only one correspond-
Fdz - Gdt (A.5)
ing charactenstic. Physically, this 1s reasonable, because that means that
Fdw - Hdt there can only be a smgle composition at any point and time.
Hdz - Gdw By their nature, however, charactenstics that represent different composi-
Most useful applicattons of pressure swmg adsorption have auasilinear tions have different slopes. Generally, those havmg greater amounts of the
matenal balance equations, due to the dependence of both velocity and the more strongly adsorbed comoonent have larger slopes, as shown m Ea. A.6.
adsorption isotherm on partial pressure. In fact, Eq. A.1 1s eau1valent to a Thus when the influent contams more of the more strongly adsorbed compo-
continuity equation of component t for a binary mixture in a fixed bed. nent than the initial column contents, the charactenst1cs of the influent
Furthermore. the approoriate form can be denvect from Ea. 4.4, by applying would tend to ovcriap those of the initial contents. That, as 'ment1oned,,
the chain rule for differentiation with some algebraic mamouiatio·.1. Soecifi- would be 1mpossible. The conflict cannot be resolved by merely averagmg the
cally, that equation is obtained from two independent equations: the first compositions or blending the equations. Rather, an overall material balance
being for component 1 (or 2), and the second representmg the sum of is performed, looking at the sliver of adsorbent mto which passes the
comoonents I and 2. high-concentration materiai, and out of which flows the low-concentration
In the resultmg equation, w 1s taken to be the mole fract10n of comoonent matenal. In fact, 11 may be helpful to visualize the composition shift that
,, ancl F is adjusted to unity. In so clomg, G describes the conveyance due to occurs both m terms of position and time. The first illustration, Figure A. l(a)
bulk motion through the fixed beet and the distribution between the fluid anct shows composition profiles at three instants of time,_ one of which catches the
solid phases. while H relates the nature of a composition shift that corre- front m the region of mterest. The second, Figure A.J(b), shows identical
sponds to a pressure shift and includes the distribution between the fluid and data, but in the form of internal breakthrough curves (i.e., histoiies at three
solid phases. The symbolic definitions are: axial positions). The shapes of the curves do not matter (as iong as they are
not spreading); all that matters ts the composition shift. The fronts 1ri the
G - {3Au
I + ({3 - 1) Y; illustratmn are sketched as rounded, though-the cquilibnum theory considers
them to be step changes.
(/3 - 1)(1 - Y;)Y; d In P The key concept explainmg the movement of the front is that there is no
Ii -
I + (/3 - l)y 1 di accu,mulation at the front itself. This 1s equivalent to saymg that the adsorb-
Even for linear isotherms (where {3, = (1 + [(I - e)je]k,)-•, and {3 = ent m the bed is uniform, or that the isotherms are independent of ax.iai
Position. Following the sketches, 1t is most useful ;to consider elements of
{3 Al {3 8 ), the mterstitial fluid velocity depends on the amount ·of component ,
soace and time (e.g., a sliver of adsorbent and a moment of time). For that