Page 159 - Principles of Catalyst Development
P. 159
CATALYST CHARACTERIZATION 147
v =
>
ci
UJ
m
a:
0 MUL T1LAYERS
C/)
c
c(
UJ
~
::>
..J
0
>
MONOLAYER
o 0.1 0.2 0.3
P I Po
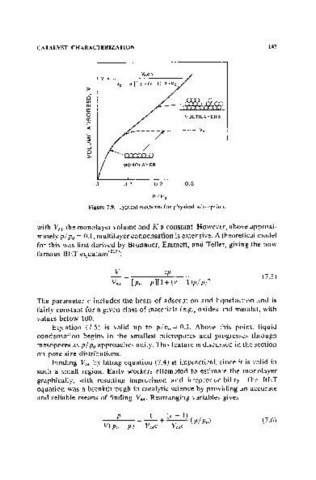
Figure 7.9. Typical isotherm for physical adsorption.
with VM the monolayer volume and K a constant. Howeve,r, above approxi-
mately pi Po = 0.1, multilayer condensation is extensive. A theoretical model
for this was first derived by Brunauer, Emmett, and Teller, giving the now
famous BET equation(215):
v cp
(7.5)
VM [Po-p][I+(c-l)plpo]
The parameter c includes the heats of adsorption and liquefaction and is
fairly constant for a given class of materials (e.g., oxides and metals), with
values below 100.
Equation (7.5) is valid up to plpo = 0.3. Above this point, liquid
condensation begins in the smallest micro pores and progresses through
mesopores as pi Po approaches unity. This feature is discussed in the section
on pore size distributions.
Finding VM by fitting equation (7.4) is impractical, s,ince it is valid in
such a small region. Early workers attempted to estimate the monolayer
graphically, with resulting imprecision and irreproducibility. The BET
equation was a breakthrough in catalytic science by providing an accurate
and reliable means of finding VM . Rearranging variables gives
(c -I) ( I )
1
P
---"-- = -- + --- p Po (7.6)
V(Po - p) VMc VMc