Page 163 - Principles of Catalyst Development
P. 163
CATALYST CHARACTERIZATION 151
METERING VALVE
CALIBRA TlON
GAS
#1 #2 INJECTION VALVE
GAS MIXTURES
CELL
'---_--J CATALYST
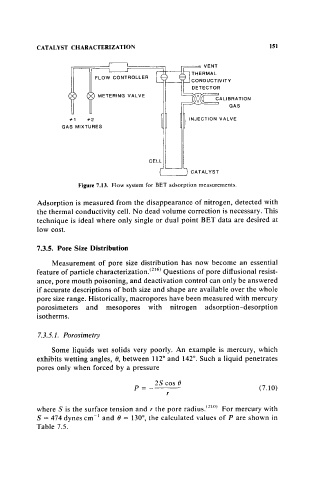
Figure 7.13. Flow system for BET adsorption measurements.
Adsorption is measured from the disappearance of nitrogen, detected with
the thermal conductivity cell. No dead volume correction is necessary. This
technique is ideal where only single or dual point BET data are desired at
low cost.
7.3.5. Pore Size Distribution
Measurement of pore size distribution has now become an essential
feature of particle characterization.(216) Questions of pore diffusional resist-
ance, pore mouth poisoning, and deactivation control can only be answered
if accurate descriptions of both size and shape are available over the whole
pore size range. Historically, macropores have been measured with mercury
porosimeters and mesopores with nitrogen adsorption-desorption
isotherms.
7.3.5.1. Porosimetry
Some liquids wet solids very poorly. An example is mercury, which
exhibits wetting angles, (), between 112° and 142°. Such a liquid penetrates
pores only when forced by a pressure
2S cos ()
P=---- (7.10)
r
where S is the surface tension and r the pore radius.(21O) For mercury with
S = 474 dynes cm -I and () = 130°, the calculated values of P are shown in
Table 7.5.