Page 241 - Process Modelling and Simulation With Finite Element Methods
P. 241
228 Process Modelling and Simulation with Finite Element Methods
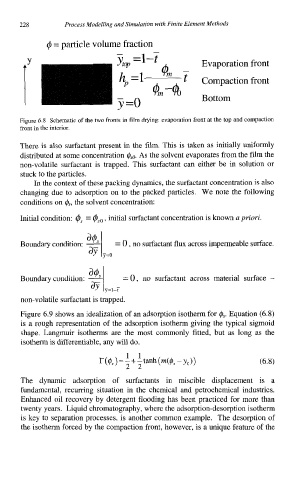
@ = particle volume fraction
yw =I-t
! - $m-h Evaporation front
V
Ym t
A
h =1
Compaction front
Bottom
y=o
Figure 6.8 Schematic of the two fronts in film drying: evaporation front at the top and compaction
front in the interior.
There is also surfactant present in the film. This is taken as initially uniformly
distributed at some concentration As the solvent evaporates from the film the
non-volatile surfactant is trapped. This surfactant can either be in solution or
stuck to the particles.
In the context of these packing dynamics, the surfactant concentration is also
changing due to adsorption on to the packed particles. We note the following
conditions on &, the solvent concentration:
Initial condition: @$ = initial surfactant concentration is known a priori.
% li-”
Boundary condition: - = 0 , no surfactant flux across impermeable surface.
Boundary condition: - = 0, no surfactant across material surface -
a@s G I jk-T
non-volatile surfactant is trapped.
Figure 6.9 shows an idealization of an adsorption isotherm for Equation (6.8)
is a rough representation of the adsorption isotherm giving the typical sigmoid
shape. Langmuir isotherms are the most commonly fitted, but as long as the
isotherm is differentiable, any will do.
The dynamic adsorption of surfactants in miscible displacement is a
fundamental, recurring situation in the chemical and petrochemical industries.
Enhanced oil recovery by detergent flooding has been practiced for more than
twenty years. Liquid chromatography, where the adsorption-desorption isotherm
is key to separation processes, is another common example. The desorption of
the isotherm forced by the compaction front, however, is a unique feature of the