Page 222 - Radiochemistry and nuclear chemistry
P. 222
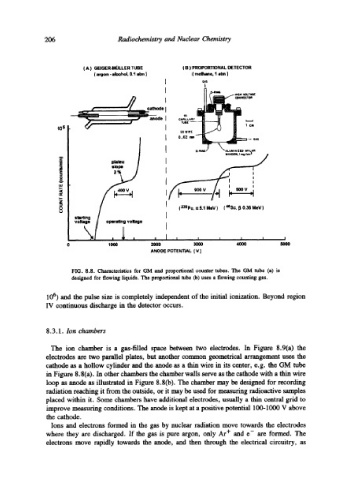
206 Radiochemistry and Nuclear Chemistry
( A ) GEIGER-MOLLER TUBE t B ) PROPORTIONAL DETECTOR
( argon -alcohol, 0.1 arm ) ( methane. 1 atm )
I MS
i
I n .0..-
II r ~ NIGH VObT~(
I "
CAIqLLARY
10 6
SS WIR(
I 0.02 m -.. GAS
WINDOW. I mg/r.ml
_c , ./
E plateu i
slope /
i 2%
UJ
I-.-
I-
z ::)
O [ (239Pu, (x 5.1 MeV ) ( ~Sc, p 0,36 MeV )
(J
starting ~ ' I
I
I! I I, I , I I I I I I
0 t000 2000 3000 4000 S000
ANODE POTENTIAL ( V ]
FIG. 8.8. Characteristics for GM and proportional counter tubes. The GM tube (a) is
designed for flowing liquids. The proportional tube (b) uses a flowing counting gas.
106 ) and the pulse size is completely independent of the initial ionization. Beyond region
IV continuous discharge in the detector occurs.
8.3.1. Ion chambers
The ion chamber is a gas-filled space between two electrodes. In Figure 8.9(a) the
electrodes are two parallel plates, but another common geometrical arrangement uses the
cathode as a hollow cylinder and the anode as a thin wire in its center, e.g. the GM tube
in Figure 8.8(a). In other chambers the chamber walls serve as the cathode with a thin wire
loop as anode as illustrated in Figure 8.8(b). The chamber may be designed for recording
radiation reaching it from the outside, or it may be used for measuring radioactive samples
placed within it. Some chambers have additional electrodes, usually a thin central grid to
improve measuring conditions. The anode is kept at a positive potential 100-1000 V above
the cathode.
Ions and electrons formed in the gas by nuclear radiation move towards the electrodes
where they are discharged. If the gas is pure argon, only Ar + and e- are formed. The
electrons move rapidly towards the anode, and then through the electrical circuitry, as