Page 223 - Radiochemistry and nuclear chemistry
P. 223
Detection and Measurement Techniques 207
@ I 2 VOLTAGE
POS IT I VE ELECTRODE ( ANODE ) HIGH
( , ) TO
VOLTMETER
NUCLEAR f
PART ! CLE
:GROUND
NEGATIVE
INSULATOR" ELECTRODE
(CATHODE)
a b
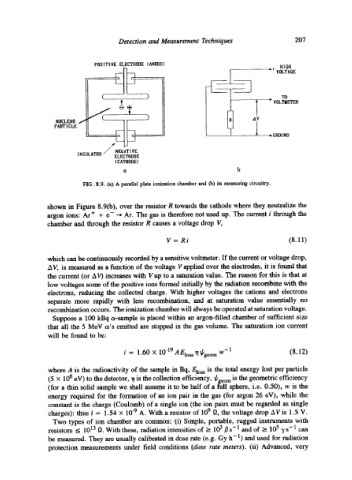
FIG. 8.9. (a) A parallel plate ionization chamber and (b) its measuring circuitry.
shown in Figure 8.9(b), over the resistor R towards the cathode where they neutralize the
argon ions: Ar + + e- ~ Ar. The gas is therefore not used up. The current i through the
chamber and through the resistor R causes a voltage drop V,
V = Ri (8.11)
which can be continuously recorded by a sensitive voltmeter. If the current or voltage drop,
AV, is measured as a function of the voltage V applied over the electrodes, it is found that
the current (or AV) increases with V up to a saturation value. The reason for this is that at
low voltages some of the positive ions formed initially by the radiation recombine with the
electrons, reducing the collected charge. With higher voltages the cations and electrons
separate more rapidly with less recombination, and at saturation value essentially no
recombination occurs. The ionization chamber will always be operated at saturation voltage.
Suppose a 100 kBq a-sample is placed within an argon-filled chamber of sufficient size
that all the 5 MeV c~'s emitted are stopped in the gas volume. The saturation ion current
will be found to be:
i = 1.60 x 10 -19 A Eloss ~/~geom w- 1 (8.12)
where A is the radioactivity of the sample in Bq, Eloss is the total energy lost per particle
(5 x 106 eV) to the detector, ~/is the collection efficiency, ~kgeom is the geometric efficiency
(for a thin solid sample we shall assume it to be half of a full sphere, i.e. 0.50), w is the
energy required for the formation of an ion pair in the gas (for argon 26 eV), while the
constant is the charge (Coulomb) of a single ion (the ion pairs must be regarded as single
charges): thus i = 1.54 x 10 -9 A. With a resistor of 109 f~, the voltage drop AV is 1.5 V.
Two types of ion chamber are common: (i) Simple, portable, rugged instruments with
resistors _ 1013 fl. With these, radiation intensities of _> 103 ~s -1 and of >_ 105 -ys -1 can
be measured. They are usually calibrated in dose rate (e.g. Gy h-1) and used for radiation
protection measurements under field conditions (dose rate meters). (ii) Advanced, very