Page 234 - Radiochemistry and nuclear chemistry
P. 234
218 Radiochemistry and Nuclear Chemistry
$AHPLI~ VIAL
TRAHSrARENT. l~\\\\\'ql-_"-.:.llk\\\\\q O~ICAL COUPLING
/
",, ~\\\\\\~\\\\~
w[NOOw
votes ~:~I ;'r- i.---;?,-
i l ":-':- ::-:-~_ ! 0
~., :'___
e:. ;;:::!,1_
10 DYNO~S .... "':
II • o
II ,~: ~::'_-I -,- 0
~':.'" . .,,i_
i , I : + VOLTAGE
,,,
~lOl~ / It _ SISAL
t~ - OUT
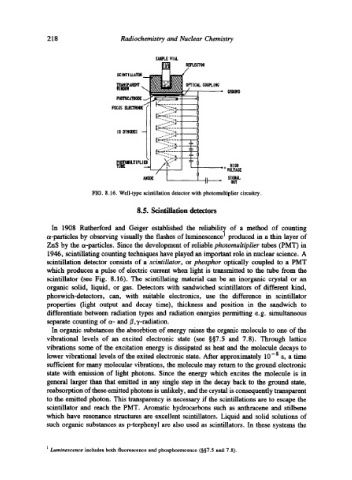
FIG. 8.16. Well-type scintillation detector with photomultiplier circuitry.
8.5. Scintillation detectors
In 1908 Rutherford and Geiger established the reliability of a method of counting
a-particles by observing visually the flashes of luminescence I produced in a thin layer of
ZnS by the a-particles. Since the development of reliable photomultiplier tubes (PMT) in
1946, scintillating counting techniques have played an important role in nuclear science. A
scintillation detector consists of a scintillator, or phosphor optically coupled to a PMT
which produces a pulse of electric current when light is transmitted to the tube from the
scintillator (see Fig. 8.16). The scintillating material can be an inorganic crystal or an
organic solid, liquid, or gas. Detectors with sandwiched scintillators of different kind,
phoswich-detectors, can, with suitable electronics, use the difference in scintillator
properties (light output and decay time), thickness and position in the sandwich to
differentiate between radiation types and radiation energies permitting e.g. simultaneous
separate counting of ce- and 3,7-radiation.
In organic substances the absorbtion of energy raises the organic molecule to one of the
vibrational levels of an excited electronic state (see {}{}7.5 and 7.8). Through lattice
vibrations some of the excitation energy is dissipated as heat and the molecule decays to
lower vibrational levels of the exited electronic state. After approximately 10 -8 s, a time
sufficient for many molecular vibrations, the molecule may return to the ground electronic
state with emission of light photons. Since the energy which excites the molecule is in
general larger than that emitted in any single step in the decay back to the ground state,
reabsorption of these emitted photons is unlikely, and the crystal is consequently transparent
to the emitted photon. This transparency is necessary if the scintillations are to escape the
scintillator and reach the PMT. Aromatic hydrocarbons such as anthracene and stilbene
which have resonance structures are excellent scintillators. Liquid and solid solutions of
such organic substances as p-terphenyl are also used as scintillators. In these systems the
1 Luminescence includes both fluorescence and phosphorescence (w167 and 7.8).