Page 235 - Radiochemistry and nuclear chemistry
P. 235
Detection and Measurement Techniques 219
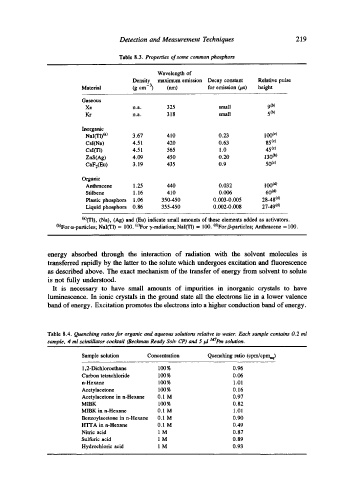
Table 8.3. Properties of some common phosphors
Wavelength of
Density maximum emission Decay constant Relative pulse
Material (g cm -3) (nm) for emission (p,s) height
Gaseous
Xe n.a. 325 small 9 (b)
Kr n.a. 318 small 5 (b)
Inorganic
NaI(TI) (a) 3.67 410 0.23 100 (c)
Csl(Na) 4.51 420 0.63 85 (c)
CsI(TI) 4.51 565 1.0 45 (c)
ZnS(Ag) 4.09 450 0.20 130 c~
CaF2(Eu ) 3.19 435 0.9 50 (c)
Organic
Anthracene 1.25 440 0.032 100 (d)
Stilbene 1.16 410 0.006 60 (d)
Plastic phosphors 1.06 350-450 0.003-0.005 28-48 (d)
Liquid phosphors 0.86 355-450 0.002-0.008 27-49 (d)
(a)(Tl), (Na), (Ag) and (Eu) indicate small amounts of these elements added as activators.
(b)For c~-particles; NaI(TI) = 100. (C)For "y-radiation; NaI(TI) = 100. (d)For/J-particles; Anthracene = 100.
energy absorbed through the interaction of radiation with the solvent molecules is
transferred rapidly by the latter to the solute which undergoes excitation and fluorescence
as described above. The exact mechanism of the transfer of energy from solvent to solute
is not fully understood.
It is necessary to have small amounts of impurities in inorganic crystals to have
luminescence. In ionic crystals in the ground state all the electrons lie in a lower valence
band of energy. Excitation promotes the electrons into a higher conduction band of energy.
Table 8.4. Quenching ratios for organic and aqueous solutions relative to water. Each sample contains O. 2 ml
sample, 4 ml scintillator cocktail (Beckman Ready Solv CP) and 5 ld ldZem solution.
Sample solution Concentration Quenching ratio (cpm/cpmaq)
1,2-Dichlorocthane 100 % 0.96
Carbon tetrachloride 100 % 0.06
n-Hexane 100% 1.01
Acetylacetone 100 % 0.16
Acetylacetone in n-Hexane 0. I M 0.97
MIBK 100% 0.82
MIBK in n-Hexane 0.I M 1.01
Benzoylacetone in n-Hexane 0. I M 0.90
HTFA in n-Hexane 0.I M 0.49
Nitric acid I M 0.87
Sulfuric acid 1 M 0.89
Hydrochloric acid 1 M 0.93