Page 191 - Reservoir Formation Damage
P. 191
Crystal Growth and Scale Formation in Porous Media 173
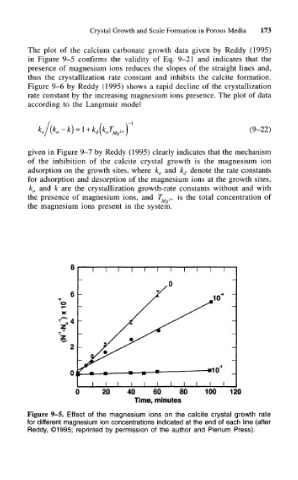
The plot of the calcium carbonate growth data given by Reddy (1995)
in Figure 9-5 confirms the validity of Eq. 9-21 and indicates that the
presence of magnesium ions reduces the slopes of the straight lines and,
thus the crystallization rate constant and inhibits the calcite formation.
Figure 9-6 by Reddy (1995) shows a rapid decline of the crystallization
rate constant by the increasing magnesium ions presence. The plot of data
according to the Langmuir model
(9-22)
given in Figure 9-7 by Reddy (1995) clearly indicates that the mechanism
of the inhibition of the calcite crystal growth is the magnesium ion
adsorption on the growth sites, where k a and k d denote the rate constants
for adsorption and desorption of the magnesium ions at the growth sites,
k 0 and k are the crystallization growth-rate constants without and with
the presence of magnesium ions, and T M 2+ is the total concentration of
the magnesium ions present in the system.
40 60 80 100 120
Time, minutes
Figure 9-5. Effect of the magnesium ions on the calcite crystal growth rate
for different magnesium ion concentrations indicated at the end of each line (after
Reddy, ©1995; reprinted by permission of the author and Plenum Press).