Page 117 - Separation process principles 2
P. 117
,
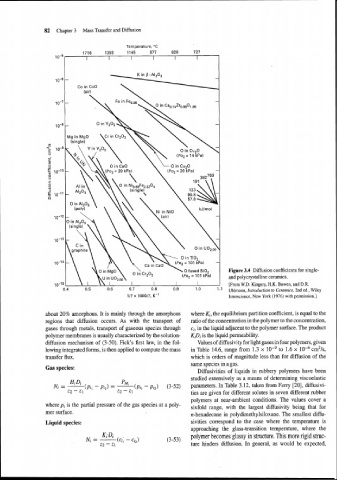
82 Chapter 3 Mass Transfer and Diffusion
Temperature, "C
17;6 13r3 11;s 9;7 878 777
Figure 3.4 Diffusion coefficients for single-
and polycrystalline ceramics.
[From W.D. Kingery, H.K. Bowen, and D.R.
Uhlmann, Introduction to Ceramics, 2nd ed., Wiley
Interscience, New York (1976) with permission.]
about 20% amorphous. It is mainly through the amorphous where Ki, the equilibrium partition coefficient, is equal to the
regions that diffusion occurs. As with the transport of ratio of the concentration in the polymer to the concentration,
gases through metals, transport of gaseous species through ci, in the liquid adjacent to the polymer surface. The product
polymer membranes is usually characterized by the solution- KiDi is the liquid permeability.
diffusion mechanism of (3-50). Fick's iirst law, in the fol- Values of diffusivity for light gases in four polymers, given
lowing integrated forms, is then applied to compute the mass in Table 14.6, range from 1.3 x to 1.6 x cm2/s,
transfer flux. which is orders of magnitude less than for diffusion of the
same species in a gas.
Gas species:
Diffusivities of liquids in rubbery polymers have been
studied extensively as a means of determining viscoelastic
Hl Dl
N, = - PM, (pll - parameters. In Table 3.12, taken from Ferry [20], diffusivi-
(pll - pi2) = - p12) (3-52)
22 - 21 22 - 21 ties are given for different solutes in seven different rubber
polymers at near-ambient conditions. The values cover a
where Pl is the partial Pressure of the gas at a PO'Y-
sixfold range, with the lugest diffusivity being that for
mer surface.
n-hexadecane in polydimethylsiloxane. The smallest diffu-
Liquid species: sivities correspond to the case where the temperature is
approaching the glass-transition temperature, where the
Ki Di polymer becomes glassy in structure. This more rigid struc-
N, = - cz2) (3-53)
-
(~1,
22 - ZI ture hinders diffusion. In general, as would be expected,