Page 76 - Separation process principles 2
P. 76
2.4 Graphical Correlations of Thermodynamic Properties 41
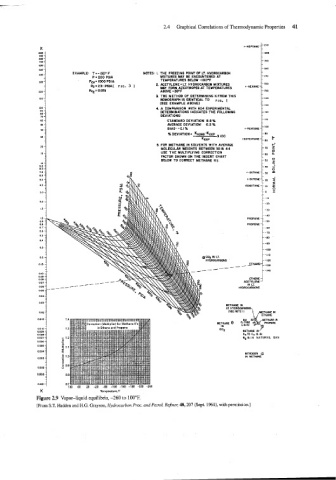
K ~-HEPTANE--'"
- 200
- 10
- 180
EXAMPLE: T =-150. F NOTES: I. THE FREEZING POINT OF LT. HYDROCARBON
P = 200 PSlA MIXTURES MAY BE ENCOUNTERED AT -170
PC". 1000 PSlA TEMPERATURES BELOW -100.E -160
PG=Z~IPSIA( FIG. 3 1 2. ACETYLENE - LT. HYDROCARBON MIXTURES
MY FORM AZEOTROPES AT TEMPERATURES
KQ - 0.051 ABOVE -35.F -150
3 THE METHOD OF DETERMINING K FROM THIS
NOMOGRAPH IS IDENTICAL TO I =, 1 - I40
(SEE EXAMPLE ABOVE)
-130
4 A COMPARISON WlTH 604 EXPERIMENTAL
DETERMINATIONS INDICATES THE FOLLOWING - 120
DEVIATIONS:
STANDARD DEVIATION: 8.8% - 110
AVERAGE DEVIATION: 6.2 %
BIAS: -al% ~-PENTANE-~'~~
f~ DEVIATION = KNOYO-KEXP - 90
k
ISOPENTANE-I.O
KEXP
5. FOR METHANE IN SOLVENTS WITH AVERAGE - I--
MOLECULAR WEIGHTS BETWEEN 30 8 44 -70
USE THE MULTIPLYING CORRECTION - 0
a
FACTOR SHOWN ON THE INSERT CHART -60
BELOW TO CORRECT METHANE Ks. -50 c3
- 5
-40 -J
- 0
~
n - ~ ~ 3O ~ m ~ ~ - -
L-BUTENE-I
20
- z
ISOBUTANE 10 a
- 0
-0 Z
- -10
---20
PROPANE
PROPENE
-110
@ CO, IN LT.
HYDROCARBONS - 120
__---- -140
ETHENE
0.06 - -------- HYDROCARBONS
0.07
ACETYLENE
LT.
IN
0.05
0.04
0.03
METHANE IN
LT. HYDROCARBONS
0.02 METHANE IN
ETHANE
C02 METHANE IN
CqTGC7 BIN
NO B l N NATUKLL GAS
NITROGEN $
IN METHANE
K Temperature,'F
Figure 2.9 Vapor-liquid equilibria, -260 to 100°F.
[From S.T. Hadden and H.G. Grayson, Hydrocarbon Proc. and Petrol. Refiner, 40,207 (Sept. 1961), with permission.]