Page 80 - Separation process principles 2
P. 80
2.5 Nonideal Thermodynamic Property Models 45
Table 2.6 Classical Integral Departure Equations of
~hemody narnics
~t a given temperature and composition, the following equations
give the effect of pressure above that for an ideal gas.
Mixture enthalpy:
c
m
L
(1) (h - hO,) = Pv- RT - m
Mixture entropy:
(2) (S -st) =/," (g)"dv-/; dv
I
T
pure-component fugacity coefficient:
Temperature, T
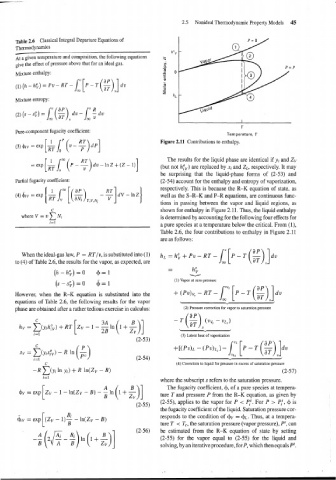
Figure 2.11 Contributions to enthalpy.
(34" =exp[&LP (v- ?)dp]
I The results for the liquid phase are identical if yi and Zv
=ex~[&--~(P- %)dv-lnZ+(Z-1) (but not hyv) are replaced by xi and ZL, respectively. It may
be surprising that the liquid-phase forms of (2-53) and
Partial fugacity coefficient: (2-54) account for the enthalpy and entropy of vaporization,
respectively. This is because the R-K equation of state, as
(4)&v=exp{&r[($) -;]dV-nz} well as the S-R-K and P-R equations, are continuous func-
T, V. N,
tions in passing between the vapor and liquid regions, as
c shown for enthalpy in Figure 2.11. Thus, the liquid enthalpy
where V = v N, is determined by accounting for the following four effects for
r=l
a pure species at a temperature below the critical. From (I),
Table 2.6, the four contributions to enthalpy in Figure 2.11
are as follows:
When the ideal-gas law, P = RT/v, is substituted into (1) hL = h; + pv - RT -
to (4) of Table 2.6, the results for the vapor, as expected, are
(s-SF) =o &= 1 (1) Vapor at zero pressure
[P-
-
T
However, when the R-K equation is substituted into the + (PV)~~ RT -lfi (g)]dv
equations of Table 2.6, the following results for the vapor L J
phase are obtained after a rather tedious exercise in calculus: (2) Pressure correction for vapor to saturation pressure
C
hv=C(yihh)+~~ ,
i=l (3) Latent heat of vaporization
(2-53)
C +[(Pv)L - (P~)L,I -
SV = Dyi,) - R (')
. A (2-54) \
i=l
~,
C (4) Correction to liquid for pressure in excess of saturation pressure
-R C(yi in yi) + R ln(Zv - B) (2-57)
r=l
where the subscript s refers to the saturation pressure.
B A ( 91
[ ture T and pressure P from the R-K equation, as given by
+v = exp Zv - 1 - ln(Zv - B) - - In 1 + - The fugacity coefficient, +, of a pure species at tempera-
(2-55) (2-55), applies to the vapor for P < P:. For P > P,S, + is
the fugacity coefficient of the liquid. Saturation pressure cor-
- Bi
I
hv = exp (Zv - 1)- - ln(Zv - B) responds to the condition of +v = +L. Thus, at a tempera-
ture T < T,, the saturation pressure (vapor pressure), PS, can
B
(2-56) be estimated from the R-K equation of state by setting
(2-55) for the vapor equal to (2-55) for the liquid and
B
-~(2~-~)ln(l+~)] solving, by an iterative procedure, for P, which then equals P.