Page 82 - Separation process principles 2
P. 82
2.6 Activity-Coefficient Models for the Liquid Phase 47
Because the reactor effluent is mostly hydrogen and methane, the
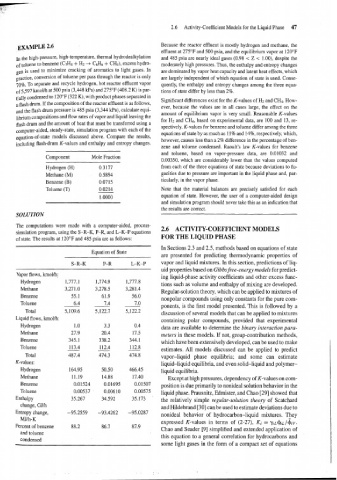
EXAMPLE 2.6
effluent at 275°F and 500 psia, and the equilibrium vapor at 120°F
ln the high-pressure, high-temperature, thermal hydrodealkylation and 485 psia are nearly ideal gases (0.98 < Z < 1.00), despite the
of toluene to benzene (C7H8 + Hz -+ C6H6 + CH4), excess hydro- moderately high pressures. Thus, the enthalpy and entropy changes
gen is used to minimize cracking of aromatics to light gases. In are dominated by vapor heat capacity and latent heat effects, which
practice, conversion of toluene per pass through the reactor is only are largely independent of which equation of state is used. Conse-
70%. To separate and recycle hydrogen, hot reactor effluent vapor quently, the enthalpy and entropy changes among the three equa-
of 5,597 kmol/h at 500 psia (3,448 kPa) and 275°F (408.2 K) is par- tions of state differ by less than 2%.
tially condensed t~ 120°F (322 K), with product phases separated in
Significant differences exist for the K-values of Hz and CH4. How-
a flash drum. If the composition of the reactor effluent is as follows,
ever, because the values are in all cases large, the effect on the
and the flash drum pressure is 485 psia (3,344 Wa), calculate equi-
amount of equilibrium vapor is very small. Reasonable K-values
librium compositions and flow rates of vapor and liquid leaving the
for H2 and CH4, based on experimental data, are 100 and 13, re-
flash drum and the amount of heat that must be transferred using a spectively. K-values for benzene and toluene differ among the three
c~mputer-aided, steady-state, simulation program with each of the
equations of state by as much as 11% and 14%, respectively, which,
equation-of-state models discussed above. Compare the results,
however, causes less than a 2% difference in the percentage of ben-
including flash-drum K-values and enthalpy and entropy changes.
zene and toluene condensed. Raoult's law K-values for benzene
and toluene, based on vapor-pressure data, are 0.01032 and
Component Mole Fraction
0.00350, which are considerably lower than the values computed
Hydrogen (H) 0.3177 from each of the three equations of state because deviations to fu-
Methane (M) 0.5894 gacities due to pressure are important in the liquid phase and, par-
Benzene (B) 0.07 15 ticularly, in the vapor phase.
Toluene (T) 0.0214 Note that the material balances are precisely satisfied for each
1 .oooo equation of state. However, the user of a computer-aided design
and simulation program should never take this as an indication that
the results are correct.
SOLUTION
The computations were made with a computer-aided, process- 2.6 ACTIVITY-COEFFICIENT MODELS
simulation program, using the S-R-K, P-R, and L-K-P equations
of state. The results at 120°F and 485 psia are as follows: FOR THE LIQUID PHASE
In Sections 2.3 and 2.5, methods based on equations of state
Equation of State
are presented for predicting thermodynamic properties of
S-R-K P-R L-K-P vapor and liquid mixtures. In this section, predictions of liq-
uid properties based on Gibbs free-energy models for predict-
Vapor flows, kmolth;
ing liquid-phase activity coefficients and other excess func-
Hydrogen
tions such as volume and enthalpy of mixing are developed.
Methane
Regular-solution theory, which can be applied to mixtures of
Benzene
nonpolar compounds using only constants for the pure com-
Toluene
ponents, is the first model presented. This is followed by a
Total
discussion of several models that can be applied to mixtures
Liquid flows, kmol/h:
containing polar compounds, provided that experimental
Hydrogen
data are available to determine the binary interaction para-
Methane
meters in these models. If not, group-contribution methods,
Benzene
which have been extensively developed, can be used to make
Toluene
estimates. All models discussed can be applied to predict
Total vapor-liquid phase equilibria; and some can estimate
K-values:
liquid-liquid equilibria, and even solid-liquid and polymer-
Hydrogen
liquid equilibria.
Methane Except at high pressures, dependency of K-values on com-
Benzene position is due primarily to nonideal solution behavior in the
Toluene liquid phase. Prausnitz, Edmister, and Chao [29] showed that
Enthalpy
the relatively simple regular-solution theory of Scatchard
change, GJIh
and Hildebrand [30] can be used to estimate deviations due to
Entropy change,
nonideal behavior of hydrocarbon-liquid mixtures. They
MJ/h-K
expressed K-values in terms of (2-27), K, = Y, L ~ L
Percent of benzene
Chao and Seader [9] simplified and extended application of
and toluene
this equation to a general correlation for hydrocarbons and
condensed
some light gases in the form of a compact set of equations