Page 242 - Standard Handbook Of Petroleum & Natural Gas Engineering
P. 242
Thermodynamics 2 15
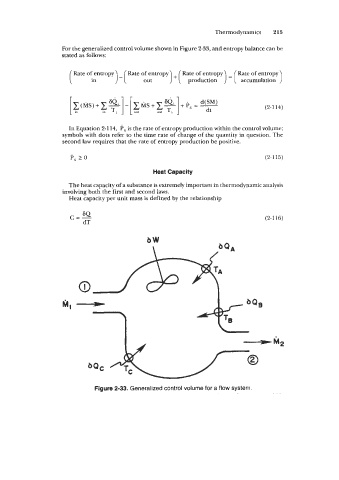
For the generalized control volume shown in Figure 2-33, and entropy balance can be
stated as follows:
( Rate of entropy )-( out ) + ( production ) = ( accumulation
Rate of entropy
Rate of entropy
Rate of entropy
in
(2-1 14)
In Equation 2-114, P, is the rate of entropy production within the control volume;
symbols with dots refer to the time rate of change of the quantity in question. The
second law requires that the rate of entropy production be positive.
P, 2 0 (2-1 15)
Heat Capacity
The heat capacity of a substance is extremely important in thermodynamic analysis
involving both the first and second laws.
Heat capacity per unit mass is defined by the relationship
C=-- SQ (2-116)
dT
Figure 2-33. Generalized control volume for a flow system.