Page 238 - Standard Handbook Of Petroleum & Natural Gas Engineering
P. 238
Thermodynamics 2 1 1
Q = A(U + PV) = H, - HA = AH [constant pressure process] (2- 108)
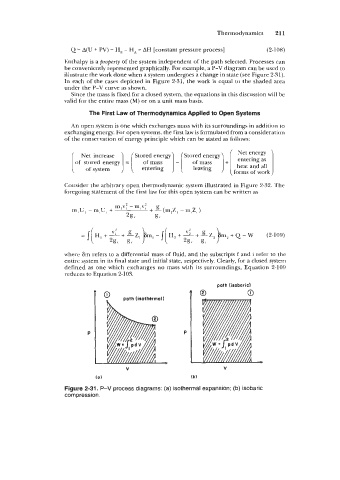
Enthalpy is a property of the system independent of the path selected. Processes can
be conveniently represented graphically. For example, a P-V diagram can be used to
illustrate the work done when a system undergoes a change in state (see Figure 2-3 1).
In each of the cases depicted in Figure 2-31, the work is equal to the shaded area
under the P-V curve as shown.
Since the mass is fixed for a closed system, the equations in this discussion will be
valid for the entire mass (M) or on a unit mass basis.
The First Law of Thermodynamics Applied to Open Systems
An open system is one which exchanges mass with its surroundings in addition to
exchanging energy. For open systems, the first law is formulated from a consideration
of the conservation of energy principle which can be stated as follows:
z;,
Net increase Stored energy Stored energy Net energy
[of stored energy) = [ of mass 1 - [ 7:s; 1 + [ ~~~~~~
of system entering
forms of work
Consider the arbitrary open thermodynamic system illustrated in Figure 2-32. The
foregoing statement of the first law for this open system can be written as
m2 + Q - W (2-109)
where 6m refers to a differential mass of fluid, and the subscripts f and i refer to the
entire system in its final state and initial state, respectively. Clearly, for a closed system
defined as one which exchanges no mass with its surroundings, Equation 2-109
reduces to Equation 2-103.
path (isobaric)
path (isothermal)
P P
V V
(0) (bl
Figure 2-31. P-V process diagrams: (a) isothermal expansion; (b) isobaric
compression.