Page 82 - Synthetic Fuels Handbook
P. 82
FUELS FROM PETROLEUM AND HEAVY OIL 69
Gas
Gas (butane and lighter)
+
Gasoline (light naphtha)
Gas Heavy naphtha
separator Kerosene
Atmospheric
Gasoline fractionation Light gas oil
Heavy gas oil
Desalter Residuum
Furnace
Crude oil
Pump
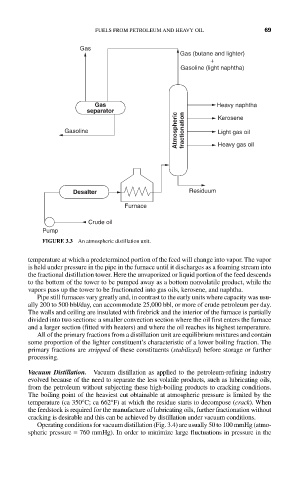
FIGURE 3.3 An atmospheric distillation unit.
temperature at which a predetermined portion of the feed will change into vapor. The vapor
is held under pressure in the pipe in the furnace until it discharges as a foaming stream into
the fractional distillation tower. Here the unvaporized or liquid portion of the feed descends
to the bottom of the tower to be pumped away as a bottom nonvolatile product, while the
vapors pass up the tower to be fractionated into gas oils, kerosene, and naphtha.
Pipe still furnaces vary greatly and, in contrast to the early units where capacity was usu-
ally 200 to 500 bbl/day, can accommodate 25,000 bbl, or more of crude petroleum per day.
The walls and ceiling are insulated with firebrick and the interior of the furnace is partially
divided into two sections: a smaller convection section where the oil first enters the furnace
and a larger section (fitted with heaters) and where the oil reaches its highest temperature.
All of the primary fractions from a distillation unit are equilibrium mixtures and contain
some proportion of the lighter constituent’s characteristic of a lower boiling fraction. The
primary fractions are stripped of these constituents (stabilized) before storage or further
processing.
Vacuum Distillation. Vacuum distillation as applied to the petroleum-refining industry
evolved because of the need to separate the less volatile products, such as lubricating oils,
from the petroleum without subjecting these high-boiling products to cracking conditions.
The boiling point of the heaviest cut obtainable at atmospheric pressure is limited by the
temperature (ca 350°C; ca 662°F) at which the residue starts to decompose (crack). When
the feedstock is required for the manufacture of lubricating oils, further fractionation without
cracking is desirable and this can be achieved by distillation under vacuum conditions.
Operating conditions for vacuum distillation (Fig. 3.4) are usually 50 to 100 mmHg (atmo-
spheric pressure = 760 mmHg). In order to minimize large fluctuations in pressure in the