Page 167 - Tandem Techniques
P. 167
Page 150
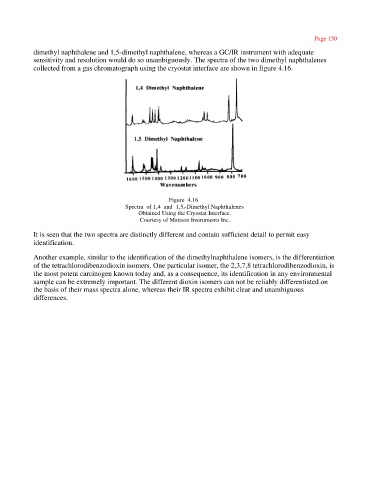
dimethyl naphthalene and 1,5-dimethyl naphthalene, whereas a GC/IR instrument with adequate
sensitivity and resolution would do so unambiguously. The spectra of the two dimethyl naphthalenes
collected from a gas chromatograph using the cryostat interface are shown in figure 4.16.
Figure 4.16
Spectra of 1,4 and 1,5,-Dimethyl Naphthalenes
Obtained Using the Cryostat Interface.
Courtesy of Mattson Instruments Inc.
It is seen that the two spectra are distinctly different and contain sufficient detail to permit easy
identification.
Another example, similar to the identification of the dimethylnaphthalene isomers, is the differentiation
of the tetrachlorodibenzodioxin isomers. One particular isomer, the 2,3,7,8 tetrachlorodibenzodioxin, is
the most potent carcinogen known today and, as a consequence, its identification in any environmental
sample can be extremely important. The different dioxin isomers can not be reliably differentiated on
the basis of their mass spectra alone, whereas their IR spectra exhibit clear and unambiguous
differences.