Page 56 - The Geological Interpretation of Well Logs
P. 56
- THE GEOLOGICAL INTERPRETATION OF WELL LOGS -
SOLIO
(non-conductive)
LIQUID (frae tons)
HYDRATION waler
sodium ion {Na }
CLAY
SOLID
schematic
walter
molecule
+ positive ion
- negalive lon
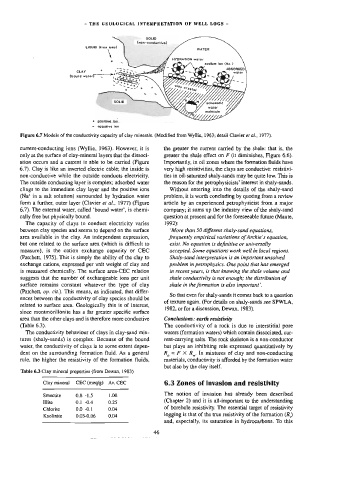
Figure 6.7 Models of the conductivity capacity of clay minerals. (Modified from Wyllie, 1963; detail Clavier et af., 1977).
current-conducting ions (Wyllie, 1963), However, it is the greater the current carried by the shale: that is, the
only at the surface of clay-mineral layers that the dissoci- greater the shale effect on F (it diminishes, Figure 6.6).
ation occurs and a current is able to be carried (Figure Importantly, in oil zones where the formation fluids have
6.7). Clay is like an inverted electric cable; the inside is very high resistivities, the clays are conductive: resistivi-
non-conductive while the outside conducts electricity. ties in oil-saturated shaly-sands may be quite low. This is
The outside conducting layer is complex; adsorbed water the reason for the petrophysicists’ interest in shaly-sands.
clings to the immediate clay layer and the positive ions Without entering into the details of the shaly-sand
(Na? in a salt solution) surrounded by hydration water problem, it is worth concluding by quoting from a review
form a further, outer layer (Clavier et al., 1977) (Figure article by an experienced petrophysicist from a major
6.7). The external water, called ‘bound water’, is chemi- comparty; it sums up the industry view of the shaly-sand
cally free but physically bound. question at present and for the foreseeable future (Maute,
The capacity of clays to conduct electricity varies 1992):
between clay species and seems to depend on the surface ‘More than 50 different shaly-sand equations,
area available in the clay. An independent expression, frequently empirical variations of Archie's equation,
but one related to the surface area (which is difficult to exist. No equation is definitive or universally
measure), is the cation exchange capacity or CEC accepted. Some equations work well in local regions.
(Patchett, 1975). This is simply the ability of the clay to Shaly-sand interpretation is an important unsolved
exchange cations, expressed per unit weight of clay and problem in petrophysics. One point that has emerged
is measured chemically. The surface area-CEC relation in recent years, is that knowing the shale volume and
suggests that the number of exchangeable ions per unit shale conductivity is not enough; the distribution of
surface remains constant whatever the type of clay shale in the formation is also important’.
(Patchett, op. cit.). This means, as indicated, that differ-
So that even for shaly-sands it comes back to a question
ences between the conductivity of clay species should be
of texture again. (For details on shaly-sands see SPWLA,
related to surface area. Geologically this is of interest,
1982, or for a discussion, Dewan, 1983).
since montmorillonite has a far greater specific surface
area than the other clays and is therefore more conductive Conclusions: earth resistivity
(Table 6.3). The conductivity of a rock is due to interstitial pore
The conductivity behaviour of clays in clay-sand mix- waters (formation waters) which contain dissociated, cur-
tures (shaly-sands) is complex. Because of the bound rent-carrying salts. The rock skeleton is a non-conductor
water, the conductivity of clays is to some extent depen- but plays an inhibiting role expressed quantitatively by
dent on the surrounding formation fluid. As a general R,= F X R,. In mixtures of clay and non-conducting
rule, the higher the resistivity of the formation fluids, materials, conductivity is afforded by the formation water
but also by the clay itself.
Table 6.3 Clay mineral properties (from Dewan, 1983)
Clay mineral CEC (meq/g) Av. CEC 6.3 Zones of invasion and resistivity
The notion of invasion has atready been described
Smectite 0.8 -1.5 1.00
(Chapter 2) and it is all-important to the understanding
Illite 0,1 -0.4 0.25
of borehole resistivity. The essential target of resistivity
Chlorite 0.0 -0.1 0.04
logging is that of the true resistivity of the formation (R,)
Kaolinite 0,03-0.06 0.04
and, especially, its saturation in hydrocarbons. To this