Page 218 - Thermal Hydraulics Aspects of Liquid Metal Cooled Nuclear Reactors
P. 218
Subchannel analysis for LMR 189
5.1.2 Liquid metals as coolant
By the selection of coolant, the following main criteria should be taken into
consideration:
– Good heat-transport properties
– Low neutron absorption or favorable neutron economics
– Chemical compatibility with fuel and structural materials
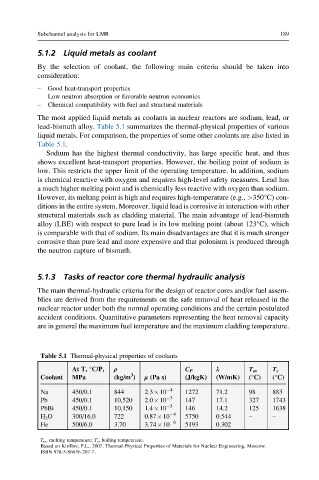
The most applied liquid metals as coolants in nuclear reactors are sodium, lead, or
lead-bismuth alloy. Table 5.1 summarizes the thermal-physical properties of various
liquid metals. For comparison, the properties of some other coolants are also listed in
Table 5.1.
Sodium has the highest thermal conductivity, has large specific heat, and thus
shows excellent heat-transport properties. However, the boiling point of sodium is
low. This restricts the upper limit of the operating temperature. In addition, sodium
is chemical reactive with oxygen and requires high-level safety measures. Lead has
a much higher melting point and is chemically less reactive with oxygen than sodium.
However, its melting point is high and requires high-temperature (e.g., >350°C) con-
ditions in the entire system. Moreover, liquid lead is corrosive in interaction with other
structural materials such as cladding material. The main advantage of lead-bismuth
alloy (LBE) with respect to pure lead is its low melting point (about 123°C), which
is comparable with that of sodium. Its main disadvantages are that it is much stronger
corrosive than pure lead and more expensive and that polonium is produced through
the neutron capture of bismuth.
5.1.3 Tasks of reactor core thermal hydraulic analysis
The main thermal-hydraulic criteria for the design of reactor cores and/or fuel assem-
blies are derived from the requirements on the safe removal of heat released in the
nuclear reactor under both the normal operating conditions and the certain postulated
accident conditions. Quantitative parameters representing the heat removal capacity
are in general the maximum fuel temperature and the maximum cladding temperature.
Table 5.1 Thermal-physical properties of coolants
ρ λ
At T, °C/P, C P T m T s
3
Coolant MPa (kg/m ) μ (Pa s) (J/kgK) (W/mK) (°C) (°C)
Na 450/0.1 844 2.3 10 4 1272 71.2 98 883
Pb 450/0.1 10,520 2.0 10 3 147 17.1 327 1743
PbBi 450/0.1 10,150 1.4 10 3 146 14.2 125 1638
H 2 O 300/16.0 722 0.87 10 4 5750 0.544 – –
He 500/6.0 3.70 3.74 10 6 5193 0.302 – –
T m , melting temperature; T s , boiling temperature.
Based on Kirillov, P.L., 2007. Thermal-Physical Properties of Materials for Nuclear Engineering. Moscow.
ISBN 978-5-86656-207-7.