Page 79 - Thermodynamics of Biochemical Reactions
P. 79
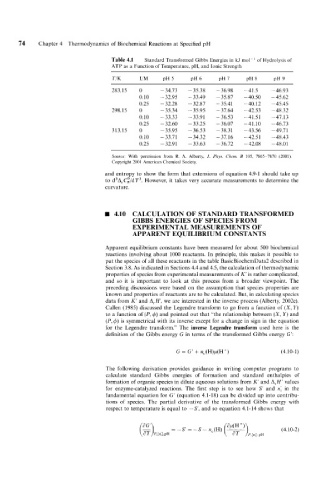
74 Chapter 4 Thermodynamics of Biochemical Reactions at Specified pH
Table 4.1 Standard Transformed Gibbs Energies in kJ mo1-I of Hydrolysis of
ATP as a Function of Temperature, pH, and Ionic Strength
283.15 0 - 34.73 - 35.38 - 36.98 -41.5 - 46.93
0.10 - 32.95 - 33.49 - 35.87 - 40.50 - 45.62
0.25 - 32.28 - 32.87 - 35.41 -40.12 - 45.45
298.15 0 - 35.34 - 35.95 - 37.64 -42.53 -48.32
0.10 - 33.33 - 33.91 - 36.53 -41.51 -47.13
0.25 - 32.60 - 33.25 - 36.07 -41.10 -46.73
313.15 0 - 35.95 - 36.53 - 38.31 - 43.56 - 49.7 1
0.10 - 33.71 - 34.32 -37.16 -42.51 -48.43
0.25 - 32.91 - 33.63 - 36.72 - 42.08 - 48.01
Source: With permission from R. A. Alberty, J. Phys. Che~fi. B 105, 7865-7870 (2001).
Copyright 2001 American Chemical Society.
and entropy to show the form that extensions of equation 4.9-1 should take up
to d3A,CF/dT3. However, it takes very accurate measurements to determine the
curvature.
4.10 CALCULATION OF STANDARD TRANSFORMED
GIBBS ENERGIES OF SPECIES FROM
EXPERIMENTAL MEASUREMENTS OF
APPARENT EQUILIBRIUM CONSTANTS
Apparent equilibrium constants have been measured for about 500 biochemical
reactions involving about 1000 reactants. In principle, this makes it possible to
put the species of all these reactants in the table BasicBiochemData2 described in
Section 3.8. As indicated in Sections 4.4 and 4.5, the calculation of thermodynamic
properties of species from experimental measurements of K‘ is rather complicated,
and so it is important to look at this process from a broader viewpoint. The
preceding discussions were based on the assumption that species properties are
known and properties of reactants are to be calculated. But, in calculating species
data from K‘ and A,H’, we are interested in the inverse process (Alberty, 2002~).
Callen (1985) discussed the Legendre transform to go from a function of (X, Y)
to a function of (P, 4) and pointed out that “the relationship between (X, Y) and
(P, 4) is symmetrical with its inverse except for a change in sign in the equation
for the Legendre transform.” The inverse Legendre transform used here is the
definition of the Gibbs energy G in terms of the transformed Gibbs energy G’:
G = G’ + n,(H)p(H+) (4.10-1)
The following derivation provides guidance in writing computer programs to
calculate standard Gibbs energies of formation and standard enthalpies of
formation of organic species in dilute aqueous solutions from K‘ and A, H‘ values
for enzyme-catalyzed reactions. The first step is to see how S’ and nI in the
fundamental equation for G‘ (equation 4.1-18) can be divided up into contribu-
tions of species. The partial derivative of the transformed Gibbs energy with
respect to temperature is equal to -S’, and so equation 4.1-14 shows that