Page 92 - Thermodynamics of Biochemical Reactions
P. 92
4.12 Plots of Thermodynamic Properties of Biochemical Reactions versus pH 87
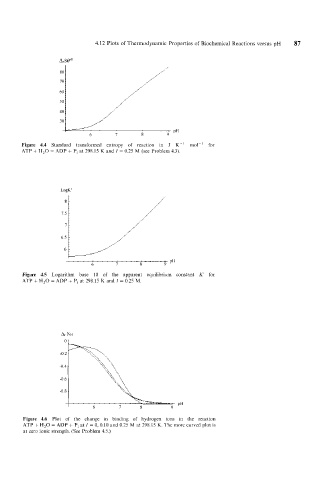
Figure 4.4 Standard transformed entropy of reaction in J K-' mo1-l for
ATP + H,O = ADP + P, at 298.15 K and I = 0.25 M (see Problem 4.3).
LogK'
. ' PH
6 7 8 9
Figure 4.5 Logarithm base 10 of the apparent equilibrium constant K' for
ATP + H,O = ADP + Pi at 298.15 K and I = 0.25 M.
Figure 4.6 Plot of the change in binding of hydrogen ions in the reaction
ATP + H,O = ADP + Pi at I = 0, 0.10 and 0.25 M at 298.15 K. The more curved plot is
at zero ionic strength. (See Problem 4.5.)