Page 87 - Thermodynamics of Biochemical Reactions
P. 87
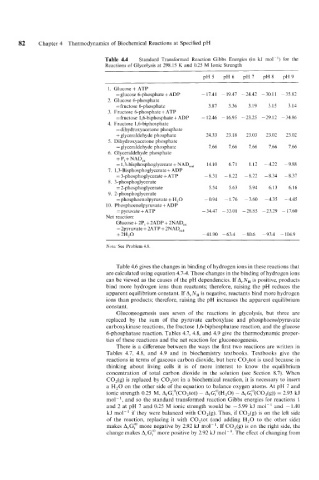
82 Chapter 4 Thermodynamics of Biochemical Reactions at Specified pH
Table 4.4 Standard Transformed Reaction Gibbs Energies (in kJ mol ') for the
Reactions of Glycolysis at 298.15 K and 0.25 M Ionic Strength
pH5 pH6 pH7 pH8 pH9
1. Glucose + ATP
=glucose 6-phosphate + ADP - 17.41 - 19.47 - 24.42 - 30.1 1 - 35.82
2. Glucose 6-phosphate
=fructose 6-phosphate 3.87 3.36 3.19 3.15 3.14
3. Fructose 6-phosphate + ATP
=fructose 1,6-biphosphate + ADP - 12.46 -16.95 -23.25 -29.12 -34.86
4. Fructose 1,6-biphosphate
= dihydroxyacetone phosphate
+ glyceraldehyde phosphate 24.33 23.18 23.03 23.02 23.02
5. Dihydroxyacetone phosphate
= gl yceraldehyde phosphatc 7.66 7.66 7.66 7.66 7.66
6. Glyceraldehyde phosphate
+ P, + NADox
= 1,3-bisphosphoglycerate + NADre, 14.10 6.71 1.12 -4.22 -9.88
7. 1,3-Bisphosphoglycerate + ADP
= 3-phosphoglycerate + ATP -8.31 -8.22 -8.22 -8.34 -8.37
8. 3-phosphoglycerate
= 2-phosphoglycerate 5.54 5.63 5.94 6.13 6.16
9. 2-phosphoglycerate
= phosphoenolpyruvate + H,O -0.94 -1.76 -3.60 -4.35 -4.45
10. Phosphoenolpyruvate + ADP
= pyruvate + ATP - 34.47 - 33.01 - 28.85 - 23.29 - 17.60
Net reaction:
Glucose + 2P, + 2ADP + 2NAD,,
= 2pyruvate + 2ATP + 2NADre,
+ 2H,O -41.90 -63.4 -80.6 -93.4 -104.9
Note: See Problem 4.8
Table 4.6 gives the changes in binding of hydrogen ions in these reactions that
are calculated using equation 4.7-4. These changes in the binding of hydrogen ions
can be viewed as the causes of the pH dependencies. If ArNH is positive, products
bind more hydrogen ions than reactants; therefore, raising the pH reduces the
apparent equilibrium constant. If ArNH is negative, reactants bind more hydrogen
ions than products; therefore, raising the pH increases the apparent equilibrium
constant.
Gluconeogenesis uses seven of the reactions in glycolysis, but three are
replaced by the sum of the pyruvate carboxylase and phosphoenolpyruvate
carboxykinase reactions, the fructose 1,6-biphosphatase reaction, and the glucose
6-phosphatase reaction. Tables 4.7, 4.8, and 4.9 give the thermodynamic proper-
ties of these reactions and the net reaction for gluconeogenesis.
There is a difference between the ways the first two reactions are written in
Tables 4.7, 4.8, and 4.9 and in biochemistry textbooks. Textbooks give the
reactions in terms of gaseous carbon dioxide, but here C0,tot is used because in
thinking about living cells it is of more interest to know the equilibrium
concentration of total carbon dioxide in the solution (see Section 8.7). When
CO,(g) is replaced by C0,tot in a biochemical reaction, it is necessary to insert
a H,O on the other side of the equation to balance oxygen atoms. At pH 7 and
ionic strength 0.25 M, A,Gio(CO,tot) - A,Gio(H,O) - AfG:'(CO,(g)) = 2.93 kJ
mol-l, and so the standard transformed reaction Gibbs energies for reactions 1
and 2 at pH 7 and 0.25 M ionic strength would be -5.99 kJ mol-l and ~ 1.40
kJ mol-' if they were balanced with CO,(g). Thus, if CO,(g) is on the left side
of the reaction, replacing it with C0,tot (and adding H,O to the other side)
makes A,GIo more negative by 2.92 kJ mol-'. If CO,(g) is on the right side, the
change makes Arc:' more positive by 2.92 kJ mol- '. The effect of changing from