Page 89 - Thermodynamics of Biochemical Reactions
P. 89
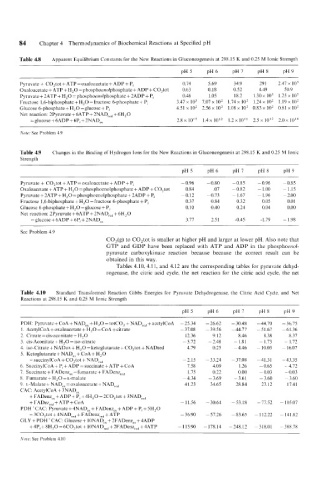
84 Chapter 4 Thermodynamics of Biochemical Reactions at Specified pH
Table 4.8 Apparent Equilibrium Constants for the New Reactions in Gluconeogenesis at 298.1 5 K and 0.25 M Ionic Strength
pH 5 pH 6 PH 7 PH 8 PH 9
Pyruvate + C0,tot + ATP = oxaloacetate + ADP + Pi 0.74 5.69 34.9 291 2.47~ 10’
H,O
Oxaloacetate + ATP ! = phosphornolphosphate + ADP + C0,tot 0.63 0.18 0.52 4.49 50.9
Pyruvate + 2ATP + H,O = phosphoenolphosphate + 2ADP t- Pi 0.46 1.05 18.2 1.30 x lo3 1.25 x 10’
Fructose 1,6-biphosphate + H,O = fructose 6-phosphate 1 P, 3.47 x 103 7.07 x 10, 1.74 x 10’ 1.24 x lo2 1.19 x 102
Glucose 6-phosphate + H,O =glucose 1 Pi 4.51 x 10, 2.56 x 10, 1.08 x 102 0.83 x 10’ 0.81 x 10’
Net reaction: 2Pyruvate + 6ATP + 2NADTe, + 6H,O
= glucose + 6ADP 1 6Pi + 2NAD,,x 2.8 x 10Is 1.4 x 1.2 x 10” 2.5 x 10” 2.0 x IOI4
Note: See Problem 4.9
Table 4.9 Changes in the Binding of Hydrogen Ions for the New Reactions in Gluconeogenesis at 298.15 K and 0.25 M Ionic
Strength
Pyruvate + C0,tot + ATP = oxaloacetate + ADP + Pi -0.96 -0.80 -0.85 -0.96 -0.85
ATP
Oxaloacetate I + H,O = phosphoenolphosphate + ADP + C0,tot 0.84 .07 -0.82 - 1.00 - 1.15
Pyruvate + 2ATP + H,O = phosphoenolphosphate + 2ADP + Pi -0.12 -0.73 - 1.67 - 1.96 -2.00
Fructose 1,6-biphosphate + H,O =fructose 6-phosphate + Pi 0.37 0.84 0.32 0.05 0.01
Glucose 6-phosphate + H,O =glucose + Pi 0.10 0.40 0.24 0.04 0.00
Net reaction: 2Pyruvate + 6ATP + 2NADrcd + 6H,O
= glucose + 6ADP + 6Pi + 2NADox 3.77 2.51 -0.45 -1.79 - 1.98
See Problem 4.9
CO,(g) to C0,tot is smaller at higher pH and larger at lower pH. Also note that
GTP and GDP have been replaced with ATP and ADP in the phosphoenol-
pyruvate carboxykinase reaction because because the correct result can be
obtained in this way.
Tables 4.10, 4.1 I, and 4.12 are the corresponding tables for pyruvate dehyd-
rogenase, the citric acid cycle, the net reaction for the citric acid cycle, the net
Table 4.10 Standard Transformed Reaction Gibbs Energies for Pyruvate Dehydrogenase, the Citric Acid Cycle, and Net
Reactions at 298.15 K and 0.25 M Ionic Strength
PDH: Pyruvatc + CoA + NADox +.H20 = totCO, + NADrcd + acetylCoA - 25.34 - 26.62 - 30.48 -44.70 - 36.75
1. AcetylCoA + oxaloacetate + H,O = CoA + citrate - 37.08 -39.56 -44.71 -51.61 -61.36
2. Citrate = cis-aconitate + H,O 12.36 9.12 8.46 8.38 8.37
3. cis-Aconitate + H,O = iso-citrate - 5.72 -2.48 - 1.81 - 1.73 - 1.72
4. iso-Citrate + NADox +H,O = ketoglutarate + C0,tot +NADred 4.79 0.25 -4.46 - 10.03 - 16.07
5. Ketoglutarate + NADOx + CoA! H,O
=succinylCoA +CO,tot +NADre, -2.15 - 33.24 - 37.08 -41.31 -43.35
+
P,
6. SuccinylCoA ! ADP = succinate + ATP + CoA 7.58 4.09 1.26 - 0.65 - 4.12
7. Succinate + FADenz,)x = fumarate + FADenzrc, 1.75 0.22 0.00 - 0.03 - 0.03
8. Fumaratc + H,O = L-malate -4.34 -3.69 -3.61 - 3.60 ~ 3.60
9. 1.-Malate 1 NADox = oxaloacetate I NADIC, 41.23 34.65 28.84 23.12 17.41
CAC: AcetylCoA + 3NADox
+ FADellzox + ADP + P, +4H,O = 2C0,tot + 3NADrc,
+ FADezre, + ATP + CoA 11.56 -30.64 -53.18 -11.52 105.07
PDH ‘CAC: Pyruvate + 4NADt,k + FADenzux + ADP + Pi + 5H,O
=3C0,tot+4NADre,+ FADenzre,+ATP - 36.90 57.26 -83.65 I 12.22 141.82
GLY + PDH’CAC: Glucose+ 10NAD~,x+2FADenzox+4ADP
+4Pi+8H,0=6C02tot + 10NADre, +2FADenzrc,+4ATP - 11 5.90 -178.14 -248.12 318.01 388.78
Now See Problem 4.10