Page 86 - Thermodynamics of Biochemical Reactions
P. 86
4.11 Tables of Standard Transformed Thermodynamic Properties at 298.15 K 81
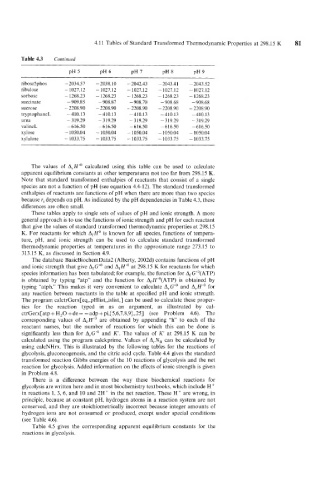
Table 4.3 Continued
ribose5phos - 2034.57 - 2038.10 - 2042.43 - 2043.41 -2043.52
ribulose - 1027.12 - 1027.12 - 1027.12 - 1027.12 - 1027.12
sorbose - 1268.23 - 1268.23 - 1268.23 - 1268.23 - 1268.23
succinate - 909.85 - 908.87 -908.70 - 908.68 -908.68
sucrose - 2208.90 - 2208.90 - 2208.90 - 2208.90 - 2208.90
tryptophaneL -410.13 -410.13 - 410.13 - 410.13 - 410.13
urea - 319.29 - 3 19.29 -319.29 - 319.29 - 319.29
valineL - 616.50 -616.50 - 616.50 -616.50 -616.50
xylose - 1050.04 - 1050.04 - 1050.04 - 1050.04 - 1050.04
x ylulose - 1033.75 - 1033.75 - 1033.75 - 1033.75 - 1033.75
The values of A,H” calculated using this table can be used to calculate
apparent equilibrium constants at other temperatures not too far from 298.15 K.
Note that standard transformed enthalpies of reactants that consist of a single
species are not a function of pH (see equation 4.4-12). The standard transformed
enthalpies of reactants are functions of pH when there are more than two species
because r, depends on pH. As indicated by the pH dependencies in Table 4.3, these
differences are often small.
These tables apply to single sets of values of pH and ionic strength. A more
general approach is to use the functions of ionic strength and pH for each reactant
that give the values of standard transformed thermodynamic properties at 298.15
K. For reactants for which A,H’ is known for all species, functions of tempera-
ture, pH, and ionic strength can be used to calculate standard transformed
thermodynamic properties at temperatures in the approximate range 273.15 to
313.15 K, as discussed in Section 4.9.
The database BasicBiochemData2 (Alberty, 2002d) contains functions of pH
and ionic strength that give A,G“ and A,H“ at 298.15 K for reactants for which
species information has been tabulated; for example, the function for Af G”(ATP)
is obtained by typing “atp” and the function for A,H”(ATP) is obtained by
typing “atph.” This makes it very convenient to calculate A,G” and A,H” for
any reaction between reactants in the table at specified pH and ionic strength.
The program calctrGerx[eq-,pHlist-,islist-] can be used to calculate these proper-
ties for the reaction typed in as an argument, as illustrated by cal-
ctrGerx[atp + H,O +de = = adp + pi,{5,6,7,8,9},.25] (see Problem 4.6). The
corresponding values of A,H“ are obtained by appending “h to each of the
reactant names, but the number of reactions for which this can be done is
significantly less than for Arc” and K‘. The values of K’ at 298.15 K can be
calculated using the program calckprime. Values of ArN, can be calculated by
using calcNHrx. This is illustrated by the following tables for the reactions of
glycolysis, gluconeogenesis, and the citric acid cycle. Table 4.4 gives the standard
transformed reaction Gibbs energies of the 10 reactions of glycolysis and the net
reaction for glycolysis. Added information on the effects of ionic strength is given
in Problem 4.8.
There is a difference between the way these biochemical reactions for
glycolysis are written here and in most biochemistry textbooks, which include H+
in reactions 1, 3, 6, and 10 and 2H’ in the net reaction. These H+ are wrong, in
principle, because at constant pH, hydrogen atoms in a reaction system are not
conserved, and they are stoichiometrically incorrect because integer amounts of
hydrogen ions are not consumed or produced, except under special conditions
(see Table 4.6).
Table 4.5 gives the corresponding apparent equilibrium constants for the
reactions in glycolysis.