Page 90 - Thermodynamics of Biochemical Reactions
P. 90
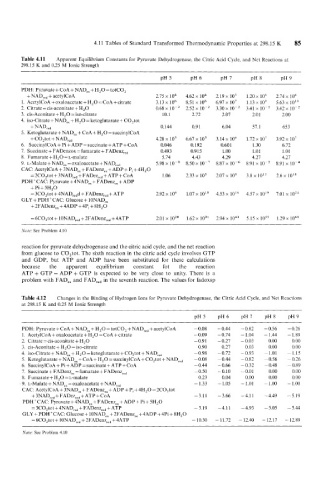
4.11 Tables of Standard Transformed Thermodynamic Properties at 298.15 K 85
Table 4.11 Apparent Equilibrium Constants for Pyruvate Dehydrogenase, the Citric Acid Cycle, and Net Reactions at
298.15 K and 0.25 M Ionic Strength
PDH: Pyruvate + CoA + NAD,, + H,O = totCO,
+ NAD,,, + acetylCoA 2.75 x 104 4.62 x 104 2.19 x 105 1.20 x 106 2.74 x lo6
1. AcetylCoA + oxaloacetate + H,O = CoA +citrate 3.13 x 10' 8.51 x lo6 6.97 x 107 1.13 109 5.63 x 10''
2. Citrate = cis-aconitate + H,O 0.68 x lo-' 2.52 x 10-2 3.30 x 10-2 3.41 x lo-' 3.42 x 10-'
3. cis-Aconitate + H,O = iso-citrate 10.1 2.72 2.07 2.01 2.00
4. iso-Citrate + NAD,, + H,O = ketoglutarate + C0,tot
+NAD,,, 0.144 0.9 1 6.04 57.1 653
5. Ketoglutarate + NAD,, + CoA + H,O = succinylCoA
+ C0,tot + NAD,,, 4.28 105 6.67 x lo5 3.14 x lo6 1.72 x 107 3.92 x 10'
6. SuccinylCoA + Pi + ADP = succinate + ATP + CoA 0.046 0.192 0.601 1.30 6.72
7. Succinate + FADenzox = fumarate + FADenq,, 0.493 0.91 5 1 .00 1.01 1.01
8. Fumarate + H,O =L-malate 5.74 4.43 4.29 4.21 4.27
9. L-Malate + NAD,, = oxaloacetate + NAD,,, 5.98 x 8.50 x 10-7 8.87 x 8.91 x 10-5 8.91 x 10-4
CAC: AcetylCoA + 3NAD,, + FADenz,, + ADP + Pi + 4H,O
= 2C0,tot + 3NAD,,, + FADez,,, + ATP + CoA 1.06 2.33 x 105 2.01 x 109 3.8 x 1013 2.6 x 1Ol8
PDH'CAC: Pyruvate + 4NAD,, + FADenz,, + ADP
+Pi + 5H,O
= 3C0,tot + 4NAD,,,d + FADenz,,, + ATP 2.92 x lo6 1.07 x 10" 4.53 x 1014 4.57 x 10'9 7.01 x 1024
GLY + PDH'CAC: Glucose + 10NAD,,
+ 2FADenz,, + 4ADP + 4Pi + 8H,O
= 6C0,tot + 10NAD,,, + 2FADenz,,, +4ATP 2.01 x 10" 1.62 x lo3' 2.94 x 5.15 x los5 1.29 x loh8
Note: See Problem 4.10
reaction for pyruvate dehydrogenase and the citric acid cycle, and the net reaction
from glucose to C0,tot. The sixth reaction in the citric acid cycle involves GTP
and GDP, but ATP and ADP have been substituted for these calculations
because the apparent equilibrium constant fot the reaction
ATP + GTP = ADP + GTP is expected to be very close to unity. There is a
problem with FAD,, and FAD,,, in the seventh reaction. The values for fadoxsp
Table 4.12 Changes in the Binding of Hydrogen Ions for Pyruvate Dehydrogenase, the Citric Acid Cycle, and Net Reactions
at 298.15 K and 0.25 M Ionic Strength
pH 5 pH6 pIX7 pH8 pH9
PDH: Pyruvate + CoA + NADox + H,O = totCO, + NAD,,, + acetylCoA ~ 0.08 - 0.44 -0.82 -0.56 -0.26
1. AcetylCoA + oxaloacetate + H,O = CoA + citrate - 0.09 - 0.74 - 1.04 - 1.44 - 1.89
2. Citrate = cis-aconitate + H,O -0.91 - 0.27 - 0.03 0.00 0.00
3. cis-Aconitate + H,O = iso-citrate 0.90 0.27 0.03 0.00 0.00
4. iso-Citrate + NADox + H,O = ketoglutarate + C0,tot + NADre, - 0.98 - 0.72 - 0.93 - 1.01 - 1.15
5. Ketoglutarate + NADox + CoA + H,O = succinylCoA + C0,tot + NADre, - 0.08 - 0.44 -0.82 -0.56 - 0.26
6. SuccinylCoA + Pi + ADP = succinate + ATP + CoA - 0.44 - 0.66 -0.32 - 0.48 -0.89
7. Succinate + FADenzox = fumarate + FADenzrC, - 0.50 -0.10 -0.01 0.00 0.00
8. Fumarate + H,O =L-malate 0.23 0.04 0.00 0.00 0.00
9. L-Malate + NADox = oxaloacetate + NADre, - 1.33 - 1.05 - 1.01 ~ 1.00 - 1.00
CAC: AcetylCoA + 3NADox + FADenzox + ADP + P, + 4H,O = 2C0,tot
+ 3NADre, + FADezre, + ATP + CoA -3.11 - 3.66 -4.11 - 4.49 -5.19
PDH'CAC: Pyruvate + 4NADox + FADenzox + ADP + Pi + 5H,O
= 3C0,tot + 4NADrc, + FADenzre, + ATP - 3.19 -4.11 - 4.93 - 5.05 - 5.44
GLY + PDHCCAC: Glucose + 10NADox + 2FADenzox + 4ADP + 4Pi + 8H,O
= 6C0,tot + 10NADre,+ 2FADenzre, + 4ATP - 10.30 - 11.72 - 12.40 - 12.17 - 12.89
Note: See Problem 4.10