Page 88 - Thermodynamics of Biochemical Reactions
P. 88
4.11 Tables of Standard Transformed Thermodynamic Properties at 298.15 K 83
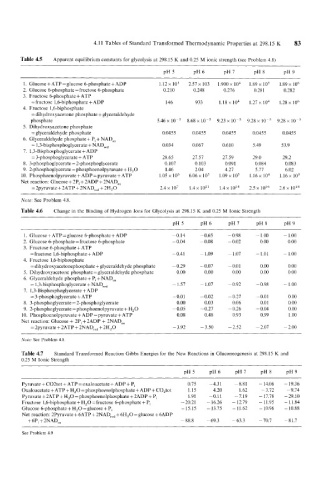
Table 4.5 Apparent equilibrium constants for glycolysis at 298.15 K and 0.25 M ionic strength (see Problem 4.8)
1. Glucose + ATP = glucose 6-phosphate + ADP 1.12 x 103 2.57 x 103 1.900 x lo4 1.89 x 10' 1.89 x 10'
2. Glucose 6-phosphate = fructose 6-phosphate 0.210 0.248 0.276 0.28 1 0.282
3. Fructose 6-phosphate + ATP
=fructose 1,6-biphosphate + ADP 146 933 1.18 x 104 1.27 x lo4 1.28 x lo6
4. Fructose 1,6-biphosphate
= dihydroxyacetone phosphate + glyceraldehyde
phosphate 5.46 x 10-5 8.68 x lo-' 9.23 x 10-5 9.28 x lo-' 9.28 x 10 -'
5. Dihydroxyacetone phosphate
= glyceraldehyde phosphate 0.0455 0.0455 0.0455 0.0455 0.0455
6. Glyceraldehyde phosphate + P, + NADc,x
= 1,3-bisphosphoglycerate +NADr,, 0.034 0.067 0.610 5.49 53.9
7. 1,3-Bisphosphoglycerate + ADP
= 3-phosphoglycerate + ATP 28.65 27.57 27.59 29.0 29.2
8. 3-phosphoglycerate = 2-phosphoglycerate 0.107 0.103 0.09 1 0.084 0.083
9. 2-phosphoglycerate = phosphoenolpyruvate + H,O 1.46 2.04 4.27 5.77 6.02
10. Phosphoenolpyruvate + ADP = pyruvate + ATP 1.05 x lo6 6.06 x 10' 1.09 x lo5 1.16 x lo4 1.16 x lo3
Net reaction: Glucose + 2Pi + 2ADP + 2NADox
= 2pyruvate + 2ATP + 2NADre, + 2H,O 2.4 x 10' 1.4 x 10" 1.4 x 2.5 x 10" 2.6 x 10l8
Note: See Problem 4.8.
Table 4.6 Change in the Binding of Hydrogen Ions for Glycolysis at 298.15 K and 0.25 M Ionic Strength
1. Glucose + ATP = glucose 6-phosphate + ADP -0.14 -0.65 - 0.98 - 1.00 - 1.00
2. Glucose 6-phosphate =fructose 6-phosphate - 0.04 - 0.08 - 0.02 0.00 0.00
3. Fructose 6-phosphate + ATP
=fructose 1,6-biphosphate + ADP -0.41 - 1.09 - 1.07 - 1.01 - 1.00
4. Fructose 1,6-biphosphate
= dihydroxyacetonephosphate + glyceraldehyde phosphate - 0.29 - 0.07 - 0.01 0.00 0.00
5. Dihydroxyacetone phosphate = glyceraldehyde phosphate 0.00 0.00 0.00 0.00 0.00
6. Glyceraldehyde phosphate + P, + NADux
= 1,3-bisphosphoglycerate + NADr,, - 1.57 - 1.07 - 0.92 -0.98 - 1.00
7. 1,3-Bisphosphoglycerate + ADP
= 3-phosphoglycerate + ATP -0.01 - 0.02 - 0.27 -0.01 0.00
8. 3-phosphoglycerate = 2-phosphoglycerate 0.00 0.03 0.06 0.01 0.00
9. 2-phosphoglycerate = phosphoenolpyruvate + H,O - 0.05 -0.21 -0.26 - 0.04 0.00
10. Phosphoenolpyruvate + ADP = pyruvate + ATP 0.08 0.48 0.93 0.99 1.00
Net reaction: Glucose + 2P, + 2ADP + 2NADox
= 2pyruvate + 2ATP + 2NADre, + 2H20 - 3.92 - 3.50 - 2.52 - 2.07 - 2.00
Note: See Problem 4.8
Table 4.7 Standard Transformed Reaction Gibbs Energies for the New Reactions in Gluconeogenesis at 298.15 K and
0.25 M Ionic Strength
Pyruvate + C02tot + ATP = oxaloacetale + ADP + P, 0.75 -4.31 -8.81 -14.06 - 19.36
Oxaloacetate + ATP + H,O = phosphoenolphosphate + ADP + C0,tot 1.15 4.20 1.62 -3.72 -9.74
Pyruvate + 2ATP + H,O = phosphoenolphosphate + 2ADP + P, 1.91 -0.11 -7.19 -17.78 -29.10
Fructose 1,6-biphosphate + H,O =fructose 6-phosphate + P, -20.21 -16.26 -12.79 -11.95 -11.84
Glucose 6-phosphate + H,O =glucose + P, - 15.15 - 13.75 - 11.62 - 10.96 - 10.88
Net reaction: 2Pyruvate + 6ATP + 2NADred + 6H20 =glucose + 6ADP
+ 6P, + 2NADox -88.8 -69.3 -63.3 -70.7 -81.7
See Problem 4.9