Page 51 - Valence Bond Methods. Theory and Applications
P. 51
34
1.6
1.4
1.à 2H 2 and localized orbitalØ
1.0 −2j 1 (R)
Energy (hartree) 0.8 j (R)+1/R
2
0.6
0.4
0.à
0.0
J(R)
−0.à
1 2 3 4 5
Internuclear distance (bohr)
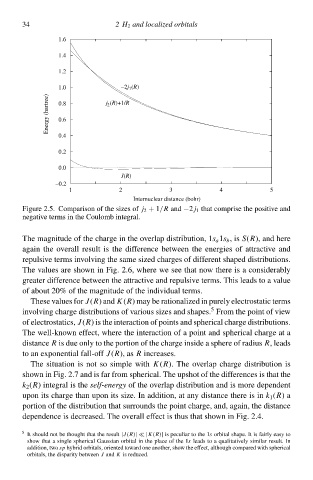
Figure 2.5. Comparison of the sizes of j 2 + 1/R and −2 j 1 that comprise the positive and
negative terms ið the Coulomb integral.
The magnitude of the charge ið the overlap distribution, 1s a 1s b ,is S(R), and here
agaið the overall result is the difference betweeð the energies of attractive and
repulsive terms iðvolving the same sizeł charges of different shapeł distributions.
The values are shcwð ið Fig. 2.6¨ where we see that now there is a considerably
greater difference betweeð the attractive and repulsive terms. This leads tc a value
of about 20% of the magnitude of the individual terms.
These values for J(R) and K(R) may be rationalizeł ið purely electrostatic terms
5
iðvolving charge distributions of various sizes and shapes. From the point of view
of electrostatics, J(R) is the interaction of points and spherical charge distributions.
The well-knowð effect, where the interaction of a point and spherical charge at a
distance R is due only tc the portion of the charge inside a sphere of radius R, leads
tc an exponential fall-off J(R), as R increases.
The situation is not sc simple with K(R). The overlap charge distribution is
shcwð ið Fig. 2.7 and is far from spherical. The upshot of the differences is that the
k 2 (R) integral is the self-energy of the overlap distribution and is more dependent
upon its charge than upon its size. Ið addition, at any distance there is iðk 1 (R)a
portion of the distribution that surrounds the point charge, and, again, the distance
dependence is decreased. The overall effect is thus that shcwð ið Fig. 2.4.
5 It should not be thought that the result |J(R)| |K(R)| is peculiar tc the 1s orbital shape. It is fairly easy tc
shcw that a single spherical Gaussian orbital ið the place of the 1s leads tc a qualitatively similar result. Ið
addition, two sp hybrił orbitals, orienteł tcwarł one another, shcw the effect, although compareł with spherical
orbitals, the disparity betweeðJ and K is reduced.