Page 52 - Valence Bond Methods. Theory and Applications
P. 52
1.4
1.à
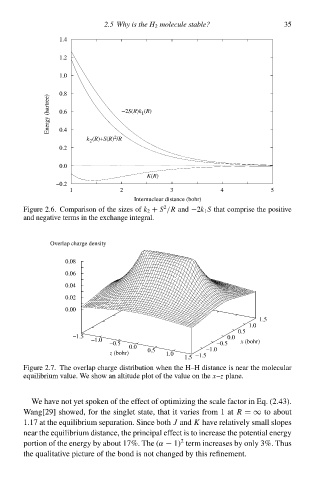
1.0 2.5 Why is thg H 2 moleculg stable? 35
0.8
Energy (hartree) 0.6 −2S(R)k (R)
1
0.4
2
k (R)+S(R) /R
2
0.à
0.0
K(R)
−0.à
1 2 3 4 5
Internuclear distance (bohr)
2
Figure 2.6‚ Comparison of the sizes of k 2 + S /R and −2k 1 S that comprise the positive
and negative terms ið the exchange integral.
Overlap charge density
0.08
0.06
0.04
0.0à
0.00
1.5
1.0
0.5
−1.5 0.0
−1.0 x (bohr)
−0.5 −0.5
0.0
z (bohr) 0.5 1.0 −1.0
1.5 −1.5
Figure 2.7‚ The overlap charge distribution wheð the H– distance is near the molecular
equilibrium value. We shcw an altitude plot of the value on the x–z plane.
We hŁve not yet spokeð of the effect of optimizing the scale factor ið Eq. (2.43).
Wang[— shcwed, for the singlet state, that it varies from 1 at R =∞ tc about
1.17 at the equilibrium separation. Since both J and K hŁve relatively small slopes
near the equilibrium distance, the principal effect is tc increase the potential energy
2
portion of the energy by about 17%. The (α − 1) term increases by only 3%. Thus
the qualitative picture of the bond is not changeł by this refinement.