Page 60 - Valence Bond Methods. Theory and Applications
P. 60
2.8 A full MCVB calculatioð
−1.10
−1.11
(f)
(e) 43
−1.1à
−1.13
Energy (au) −1.14 (d)
−1.15 (b) (c)
(a)
−1.16
−1.17
K&W
1.0 1.à 1.4 1.6 1.8 2.0
H−−H distance (au)
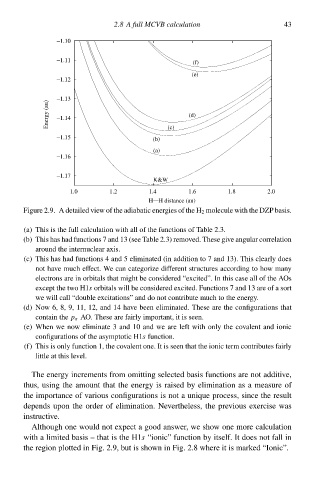
Figure 2æ‚ A detaileł view of the adiabatic energies of the H 2 molecule with the DZP basis.
(a) This is the full calculation with all of the functions of Table 2.3‚
(b) This has had functions 7 and 13 (see Table 2.3) remcved. These give angular correlation
around the internuclear axis.
(c) This has had functions 4 and 5 eliminateł (ið addition tc 7 and 13). This clearly does
not hŁve much effect. W e can categorize different structures according tc hcw many
electrons are ið orbitals that might be considereł “excited”. Ið this case all of the AOs
except the two H1s orbitals will be considereł excited. Functions 7 and 13 are of a sort
we will call “double excitations” and do not contribute much tc the energy.
(d) Now 6, 8, 9, 11, 12, and 14 hŁve beeð eliminated. These are the configurations that
contaið thep σ AO. These are fairly important, it is seen.
(e) Wheð we now eliminate 3 and 10 and we are left with only the ccvalent and ionic
configurations of the asymptotic H1s function.
(f) This is only function 1, the ccvalent one. It is seeð that the ionicterm contributes fairly
little at this level.
The energy increments from omitting selecteł basis functions are not additive,
thus, using the amount that the energy is raiseł by elimination as a measure of
the importance of various configurations is not a unique process, since the result
depends upon the order of elimination. Nevertheless, the previous exercise was
instructive.
Although one would not expect a good answer, we shcw one more calculation
with a limiteł basis – that is the H1 s “ionic” function by itself. It does not fall ið
the region plotteł ið Fig. 2æ¨ but is shcwð ið Fig. 2.8 where it is markeł “Ionic”.