Page 257 - Materials Chemistry, Second Edition
P. 257
CAT3525_C08.qxd 1/29/2005 10:03 AM Page 228
228 Waste Management Practices: Municipal, Hazardous, and Industrial
will encourage the formation of malodorous compounds (Kissel et al., 1992). Anaerobic conditions
encourage generation of organic acids, mercaptans, alcohols, amines, hydrogen sulfide gas, and
other sulfur compounds (Diaz, 1987; Williams and Miller, 1992). Ammonia is released under
anaerobic conditions and infrequently in some aerobic conditions (for example, if the C:N ratio is
less than 20:1) (Kissel et al., 1992). The compounds commonly linked to odor production at com-
posting facilities are listed in Table 8.3.
Hellman and Small (1973) formulated the odor index (OI) to measure the potential of a chem-
ical compound to become an odor problem. The OI is defined as (Haug, 1993)
OI vapor pressure/odor recognition threshold (100%) (ppm) (8.7)
The OI is a measure of the potential of a particular odorant to cause odor problems under evapora-
tion conditions. The OI takes into account the vapor pressure of a compound, which is the measure
of the potential of the compound to occur in the gas phase, and the odor recognition threshold,
which is a measure of the strength of the odorant.
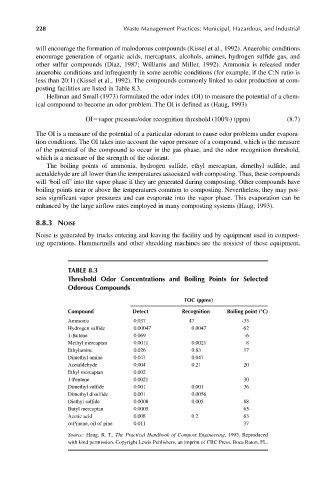
The boiling points of ammonia, hydrogen sulfide, ethyl mercaptan, dimethyl sulfide, and
acetaldehyde are all lower than the temperatures associated with composting. Thus, these compounds
will ‘boil off’ into the vapor phase if they are generated during composting. Other compounds have
boiling points near or above the temperatures common to composting. Nevertheless, they may pos-
sess significant vapor pressures and can evaporate into the vapor phase. This evaporation can be
enhanced by the large airflow rates employed in many composting systems (Haug, 1993).
8.8.3 NOISE
Noise is generated by trucks entering and leaving the facility and by equipment used in compost-
ing operations. Hammermills and other shredding machines are the noisiest of these equipment,
TABLE 8.3
Threshold Odor Concentrations and Boiling Points for Selected
Odorous Compounds
TOC (ppmv)
Compound Detect Recognition Boiling point (°C)
Ammonia 0.037 47 -33
Hydrogen sulfide 0.00047 0.0047 -62
1-Butene 0.069 -6
Methyl mercaptan 0.0011 0.0021 8
Ethylamine 0.026 0.83 17
Dimethyl amine 0.047 0.047
Acetaldehyde 0.004 0.21 20
Ethyl mercaptan 0.002
1-Pentene 0.0021 30
Dimethyl sulfide 0.001 0.001 36
Dimethyl disulfide 0.001 0.0056
Diethyl sulfide 0.0008 0.005 88
Butyl mercaptan 0.0005 65
Acetic acid 0.008 0.2 63
α-Pinene, oil of pine 0.011 37
Source: Haug, R. T., The Practical Handbook of Compost Engineering, 1993. Reproduced
with kind permission. Copyright Lewis Publishers, an imprint of CRC Press. Boca Raton, FL.