Page 310 - Water and wastewater engineering
P. 310
LIME–SODA SOFTENING 7-27
0
2
4 FeOH Fe(OH) (s)
2
log[species] 6
8
10
Fe(OH) 3 Fe 2
12
14
0 2 4 6 8 10 12 14
pH
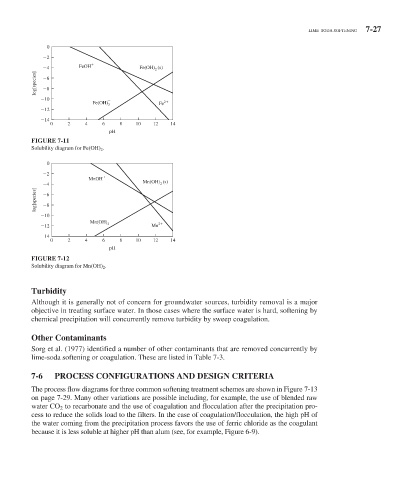
FIGURE 7-11
Solubility diagram for Fe(OH) 2 .
0
2
MnOH
2
4 Mn(OH) (s)
log[species] 6
8
10
Mn(OH)
12 3 Mn 2
14
0 2 4 6 8 10 12 14
pH
FIGURE 7-12
Solubility diagram for Mn(OH) 2 .
Turbidity
Although it is generally not of concern for groundwater sources, turbidity removal is a major
objective in treating surface water. In those cases where the surface water is hard, softening by
chemical precipitation will concurrently remove turbidity by sweep coagulation.
Other Contaminants
Sorg et al. (1977) identified a number of other contaminants that are removed concurrently by
lime-soda softening or coagulation. These are listed in Table 7-3 .
7 -6 PROCESS CONFIGURATIONS AND DESIGN CRITERIA
The process flow diagrams for three common softening treatment schemes are shown in Figure 7-13
on page 7-29 . Many other variations are possible including, for example, the use of blended raw
water CO 2 to recarbonate and the use of coagulation and flocculation after the precipitation pro-
cess to reduce the solids load to the filters. In the case of coagulation/flocculation, the high pH of
the water coming from the precipitation process favors the use of ferric chloride as the coagulant
because it is less soluble at higher pH than alum (see, for example, Figure 6-9).