Page 311 - Water and wastewater engineering
P. 311
7-28 WATER AND WASTEWATER ENGINEERING
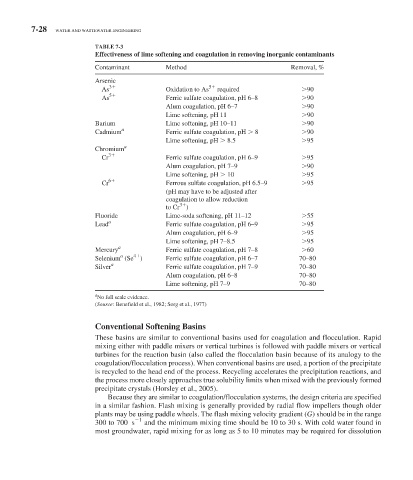
TABLE 7-3
Effectiveness of lime softening and coagulation in removing inorganic contaminants
Contaminant Method Removal, %
Arsenic
As 3 Oxidation to As 5 required 90
As 5 Ferric sulfate coagulation, pH 6–8 90
Alum coagulation, pH 6–7 90
Lime softening, pH 11 90
Barium Lime softening, pH 10–11 90
Cadmium a Ferric sulfate coagulation, pH 8 90
Lime softening, pH 8.5 95
Chromium a
Cr 3 Ferric sulfate coagulation, pH 6–9 95
Alum coagulation, pH 7–9 90
Lime softening, pH 10 95
6
Cr Ferrous sulfate coagulation, pH 6.5–9 95
(pH may have to be adjusted after
coagulation to allow reduction
3
to Cr )
Fluoride Lime-soda softening, pH 11–12 55
Lead a Ferric sulfate coagulation, pH 6–9 95
Alum coagulation, pH 6–9 95
Lime softening, pH 7–8.5 95
a
Mercury Ferric sulfate coagulation, pH 7–8 60
a 4
Selenium (Se ) Ferric sulfate coagulation, pH 6–7 70–80
a
Silver Ferric sulfate coagulation, pH 7–9 70–80
Alum coagulation, pH 6–8 70–80
Lime softening, pH 7–9 70–80
a
No full scale evidence.
(Source: Benefield et al., 1982; Sorg et al., 1977)
Conventional Softening Basins
These basins are similar to conventional basins used for coagulation and flocculation. Rapid
mixing either with paddle mixers or vertical turbines is followed with paddle mixers or vertical
turbines for the reaction basin (also called the flocculation basin because of its analogy to the
coagulation/flocculation process). When conventional basins are used, a portion of the precipitate
is recycled to the head end of the process. Recycling accelerates the precipitation reactions, and
the process more closely approaches true solubility limits when mixed with the previously formed
precipitate crystals (Horsley et al., 2005).
Because they are similar to coagulation/flocculation systems, the design criteria are specified
in a similar fashion. Flash mixing is generally provided by radial flow impellers though older
plants may be using paddle wheels. The flash mixing velocity gradient ( G ) should be in the range
1
300 to 700 s and the minimum mixing time should be 10 to 30 s. With cold water found in
most groundwater, rapid mixing for as long as 5 to 10 minutes may be required for dissolution