Page 172 - Wire Bonding in Microelectronics
P. 172
Gold-Aluminum Intermetallic Compounds 149
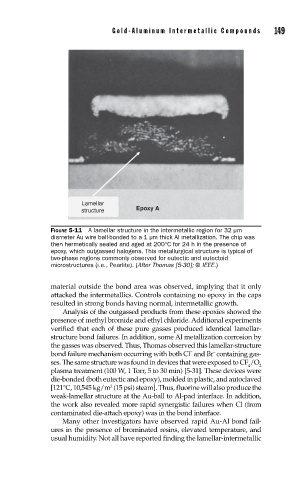
Lamellar
structure Epoxy A
FIGURE 5-11 A lamellar structure in the intermetallic region for 32 µm
diameter Au wire ball-bonded to a 1 µm thick Al metallization. The chip was
then hermetically sealed and aged at 200°C for 24 h in the presence of
epoxy, which outgassed halogens. This metallurgical structure is typical of
two-phase regions commonly observed for eutectic and eutectoid
microstructures (i.e., Pearlite). (After Thomas [5-30]; © IEEE.)
material outside the bond area was observed, implying that it only
attacked the intermetallics. Controls containing no epoxy in the caps
resulted in strong bonds having normal, intermetallic growth.
Analysis of the outgassed products from these epoxies showed the
presence of methyl bromide and ethyl chloride. Additional experiments
verified that each of these pure gasses produced identical lamellar-
structure bond failures. In addition, some Al metallization corrosion by
the gasses was observed. Thus, Thomas observed this lamellar-structure
− −
bond failure mechanism occurring with both Cl and Br containing gas-
ses. The same structure was found in devices that were exposed to CF /O
4 2
plasma treatment (100 W, 1 Torr, 5 to 30 min) [5-31]. These devices were
die-bonded (both eutectic and epoxy), molded in plastic, and autoclaved
2
[121°C, 10,545 kg/m (15 psi) steam]. Thus, fluorine will also produce the
weak-lamellar structure at the Au-ball to Al-pad interface. In addition,
the work also revealed more rapid synergistic failures when Cl (from
contaminated die-attach epoxy) was in the bond interface.
Many other investigators have observed rapid Au-Al bond fail-
ures in the presence of brominated resins, elevated temperature, and
usual humidity. Not all have reported finding the lamellar-intermetallic