Page 169 - Wire Bonding in Microelectronics
P. 169
146 Cha pte r F i v e
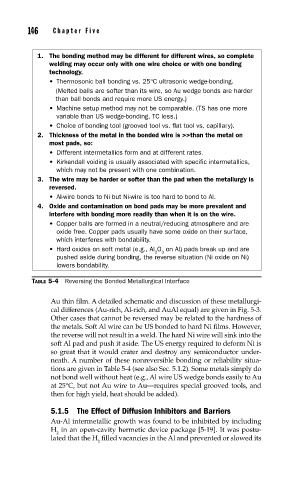
1. The bonding method may be different for different wires, so complete
welding may occur only with one wire choice or with one bonding
technology.
• Thermosonic ball bonding vs. 25°C ultrasonic wedge-bonding.
(Melted balls are softer than its wire, so Au wedge bonds are harder
than ball bonds and require more US energy.)
• Machine setup method may not be comparable. (TS has one more
variable than US wedge-bonding, TC less.)
• Choice of bonding tool (grooved tool vs. flat tool vs. capillary).
2. Thickness of the metal in the bonded wire is >>than the metal on
most pads, so:
• Different intermetallics form and at different rates.
• Kirkendall voiding is usually associated with specific intermetallics,
which may not be present with one combination.
3. The wire may be harder or softer than the pad when the metallurgy is
reversed.
• Al-wire bonds to Ni but Ni-wire is too hard to bond to Al.
4. Oxide and contamination on bond pads may be more prevalent and
interfere with bonding more readily than when it is on the wire.
• Copper balls are formed in a neutral/reducing atmosphere and are
oxide free. Copper pads usually have some oxide on their surface,
which interferes with bondability.
• Hard oxides on soft metal (e.g., Al O on Al) pads break up and are
2 3
pushed aside during bonding, the reverse situation (Ni oxide on Ni)
lowers bondability.
TABLE 5-4 Reversing the Bonded Metallurgical Interface
Au thin film. A detailed schematic and discussion of these metallurgi-
cal differences (Au-rich, Al-rich, and AuAl equal) are given in Fig. 5-3.
Other cases that cannot be reversed may be related to the hardness of
the metals. Soft Al wire can be US bonded to hard Ni films. However,
the reverse will not result in a weld. The hard Ni wire will sink into the
soft Al pad and push it aside. The US energy required to deform Ni is
so great that it would crater and destroy any semiconductor under-
neath. A number of these nonreversible bonding or reliability situa-
tions are given in Table 5-4 (see also Sec. 5.1.2). Some metals simply do
not bond well without heat (e.g., Al wire US wedge bonds easily to Au
at 25°C, but not Au wire to Au—requires special grooved tools, and
then for high yield, heat should be added).
5.1.5 The Effect of Diffusion Inhibitors and Barriers
Au-Al intermetallic growth was found to be inhibited by including
H in an open-cavity hermetic device package [5-19]. It was postu-
2
lated that the H filled vacancies in the Al and prevented or slowed its
2