Page 166 - Wire Bonding in Microelectronics
P. 166
Gold-Aluminum Intermetallic Compounds 143
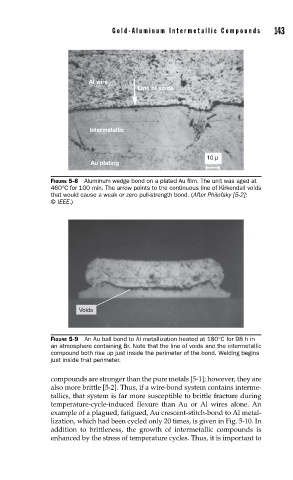
Al wire
Line of voids
Intermetallic
10 µ
Au plating
FIGURE 5-8 Aluminum wedge bond on a plated Au fi lm. The unit was aged at
460°C for 100 min. The arrow points to the continuous line of Kirkendall voids
that would cause a weak or zero pull-strength bond. (After Philofsky [5-2];
© IEEE.)
Voids
FIGURE 5-9 An Au ball bond to Al metallization heated at 180°C for 98 h in
an atmosphere containing Br. Note that the line of voids and the intermetallic
compound both rise up just inside the perimeter of the bond. Welding begins
just inside that perimeter.
compounds are stronger than the pure metals [5-1]; however, they are
also more brittle [5-2]. Thus, if a wire-bond system contains interme-
tallics, that system is far more susceptible to brittle fracture during
temperature-cycle-induced flexure than Au or Al wires alone. An
example of a plagued, fatigued, Au crescent-stitch-bond to Al metal-
lization, which had been cycled only 20 times, is given in Fig. 5-10. In
addition to brittleness, the growth of intermetallic compounds is
enhanced by the stress of temperature cycles. Thus, it is important to