Page 161 - Wire Bonding in Microelectronics
P. 161
138 Cha pte r F i v e
available (the more defects, the faster diffusion proceeds). Aluminum
and other metals diffuse very rapidly into Au by grain-boundary dif-
fusion. A discussion of this is given in Sec. 6A.4. It should be noted that
the specific intermetallic compounds in a bond-interface area are
related to the relative amounts of Au and Al present and can be differ-
ent if Al metallization contains Cu or Si in the 1 to 2% level. In addition,
some compounds may be absent because of a low nucleation probabil-
ity (they do not get started) or they may grow very slowly and are not
observed.
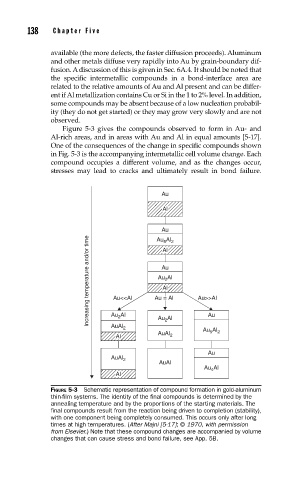
Figure 5-3 gives the compounds observed to form in Au- and
Al-rich areas, and in areas with Au and Al in equal amounts [5-17].
One of the consequences of the change in specific compounds shown
in Fig. 5-3 is the accompanying intermetallic cell volume change. Each
compound occupies a different volume, and as the changes occur,
stresses may lead to cracks and ultimately result in bond failure.
Au
Al
Au 2
Increasing temperature and/or time Au<<Al Au = Al Au>>Al
Au Al
5
Al
Au
Au Al
2
Al
Au Al
Au
2
Au Al
2
AuAl
2
Au Al 2
5
Al AuAl 2
Au
AuAl 2
AuAl
Al
Au 4
Al
FIGURE 5-3 Schematic representation of compound formation in gold-aluminum
thin-fi lm systems. The identity of the fi nal compounds is determined by the
annealing temperature and by the proportions of the starting materials. The
fi nal compounds result from the reaction being driven to completion (stability),
with one component being completely consumed. This occurs only after long
times at high temperatures. (After Majni [5-17]; © 1970, with permission
from Elsevier.) Note that these compound changes are accompanied by volume
changes that can cause stress and bond failure, see App. 5B.