Page 156 - Wire Bonding in Microelectronics
P. 156
134 Cha pte r F i v e
6 Au Al 2
Layer thickness, x (cm × 10 –3 ) 4 Au 2 Al
5
(Tan)
(Tan)
2
AuAl 2 AuAl
(Purple) (White)
Au Al
4
(Tan)
0
100 200
t [(s) 1/2 ]
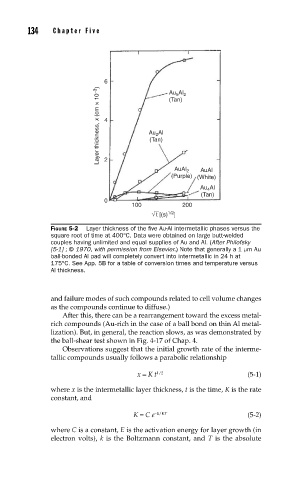
FIGURE 5-2 Layer thickness of the fi ve Au-Al intermetallic phases versus the
square root of time at 400°C. Data were obtained on large butt-welded
couples having unlimited and equal supplies of Au and Al. (After Philofsky
[5-1] ; © 1970, with permission from Elsevier.) Note that generally a 1 µm Au
ball-bonded Al pad will completely convert into intermetallic in 24 h at
175°C. See App. 5B for a table of conversion times and temperature versus
Al thickness.
and failure modes of such compounds related to cell volume changes
as the compounds continue to diffuse.)
After this, there can be a rearrangement toward the excess metal-
rich compounds (Au-rich in the case of a ball bond on thin Al metal-
lization). But, in general, the reaction slows, as was demonstrated by
the ball-shear test shown in Fig. 4-17 of Chap. 4.
Observations suggest that the initial growth rate of the interme-
tallic compounds usually follows a parabolic relationship
x = K t 1/2 (5-1)
where x is the intermetallic layer thickness, t is the time, K is the rate
constant, and
K = C e −E/KT (5-2)
where C is a constant, E is the activation energy for layer growth (in
electron volts), k is the Boltzmann constant, and T is the absolute