Page 265 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 265
P2: KVU/KXT
QC: —/—
T1: IML
P1: KVU/KXT
20:46
AT029-Manual-v7.cls
June 22, 2007
AT029-06
AT029-Manual
6. THERMODYNAMIC RELATIONS FOR PROPERTY ESTIMATIONS 245
(1)
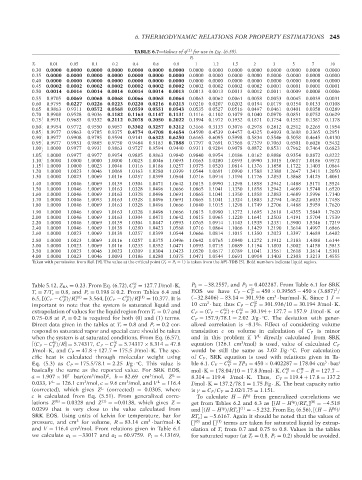
TABLE 6.7—Values of φ
P r for use in Eq. (6.59).
T r 0.01 0.05 0.1 0.2 0.4 0.6 0.8 1 1.2 1.5 2 3 5 7 10
0.30 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
0.35 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
0.40 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000 0.0000
0.45 0.0002 0.0002 0.0002 0.0002 0.0002 0.0002 0.0002 0.0002 0.0002 0.0002 0.0002 0.0001 0.0001 0.0001 0.0001
0.50 0.0014 0.0014 0.0014 0.0014 0.0014 0.0014 0.0013 0.0013 0.0013 0.0013 0.0012 0.0011 0.0009 0.0008 0.0006
0.55 0.9705 0.0069 0.0068 0.0068 0.0066 0.0065 0.0064 0.0063 0.0062 0.0061 0.0058 0.0053 0.0045 0.0039 0.0031
0.60 0.9795 0.0227 0.0226 0.0223 0.0220 0.0216 0.0213 0.0210 0.0207 0.0202 0.0194 0.0179 0.0154 0.0133 0.0108
0.65 0.9863 0.9311 0.0572 0.0568 0.0559 0.0551 0.0543 0.0535 0.0527 0.0516 0.0497 0.0461 0.0401 0.0350 0.0289
0.9908 0.9528 0.9036 0.1182 0.1163 0.1147 0.1131 0.1116 0.1102 0.1079 0.1040 0.0970 0.0851 0.0752 0.0629
0.75 0.9931 0.9683 0.9332 0.2112 0.2078 0.2050 0.2022 0.1994 0.1972 0.1932 0.1871 0.1754 0.1552 0.1387 0.1178
0.80 0.9954 0.9772 0.9550 0.9057 0.3302 0.3257 0.3212 0.3168 0.3133 0.3076 0.2978 0.2812 0.2512 0.2265 0.1954
0.85 0.9977 0.9863 0.9705 0.9375 0.4774 0.4708 0.4654 0.4590 0.4539 0.4457 0.4325 0.4093 0.3698 0.3365 0.2951
0.90 0.9977 0.9908 0.9795 0.9594 0.9141 0.6323 0.6250 0.6165 0.6095 0.5998 0.5834 0.5546 0.5058 0.4645 0.4130
0.95 0.9977 0.9931 0.9885 0.9750 0.9484 0.9183 0.7888 0.7797 0.7691 0.7568 0.7379 0.7063 0.6501 0.6026 0.5432
1.00 1.0000 0.9977 0.9931 0.9863 0.9727 0.9594 0.9440 0.9311 0.9204 0.9078 0.8872 0.8531 0.7962 0.7464 0.6823
1.05 1.0000 0.9977 0.9977 0.9954 0.9885 0.9863 0.9840 0.9840 0.9954 1.0186 1.0162 0.9886 0.9354 0.8872 0.8222
1.10 1.0000 1.0000 1.0000 1.0000 1.0023 1.0046 1.0093 1.0163 1.0280 1.0593 1.0990 1.1015 1.0617 1.0186 0.9572
1.15 1.0000 1.0000 1.0023 1.0046 1.0116 1.0186 1.0257 1.0375 1.0520 1.0814 1.1376 1.1858 1.1722 1.1403 1.0864
0.70 --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
1.20 1.0000 1.0023 1.0046 1.0069 1.0163 1.0280 1.0399 1.0544 1.0691 1.0990 1.1588 1.2388 1.2647 1.2411 1.2050
1.30 1.0000 1.0023 1.0069 1.0116 1.0257 1.0399 1.0544 1.0716 1.0914 1.1194 1.1776 1.2853 1.3868 1.4125 1.4061
1.40 1.0000 1.0046 1.0069 1.0139 1.0304 1.0471 1.0642 1.0815 1.0990 1.1298 1.1858 1.2942 1.4488 1.5171 1.5524
1.50 1.0000 1.0046 1.0069 1.0163 1.0328 1.0496 1.0666 1.0865 1.1041 1.1350 1.1858 1.2942 1.4689 1.5740 1.6520
1.60 1.0000 1.0046 1.0069 1.0163 1.0328 1.0496 1.0691 1.0865 1.1041 1.1350 1.1858 1.2883 1.4689 1.5996 1.7140
1.70 1.0000 1.0046 1.0093 1.0163 1.0328 1.0496 1.0691 1.0865 1.1041 1.1324 1.1803 1.2794 1.4622 1.6033 1.7458
1.80 1.0000 1.0046 1.0069 1.0163 1.0328 1.0496 1.0666 1.0840 1.1015 1.1298 1.1749 1.2706 1.4488 1.5959 1.7620
1.90 1.0000 1.0046 1.0069 1.0163 1.0328 1.0496 1.0666 1.0815 1.0990 1.1272 1.1695 1.2618 1.4355 1.5849 1.7620
2.00 1.0000 1.0046 1.0069 1.0163 1.0304 1.0471 1.0642 1.0815 1.0965 1.1220 1.1641 1.2503 1.4191 1.5704 1.7539
2.20 1.0000 1.0046 1.0069 1.0139 1.0304 1.0447 1.0593 1.0765 1.0914 1.1143 1.1535 1.2331 1.3900 1.5346 1.7219
2.40 1.0000 1.0046 1.0069 1.0139 1.0280 1.0423 1.0568 1.0716 1.0864 1.1066 1.1429 1.2190 1.3614 1.4997 1.6866
2.60 1.0000 1.0023 1.0069 1.0139 1.0257 1.0399 1.0544 1.0666 1.0814 1.1015 1.1350 1.2023 1.3397 1.4689 1.6482
2.80 1.0000 1.0023 1.0069 1.0116 1.0257 1.0375 1.0496 1.0642 1.0765 1.0940 1.1272 1.1912 1.3183 1.4388 1.6144
3.00 1.0000 1.0023 1.0069 1.0116 1.0233 1.0352 1.0471 1.0593 1.0715 1.0889 1.1194 1.1803 1.3002 1.4158 1.5813
3.50 1.0000 1.0023 1.0046 1.0023 1.0209 1.0304 1.0423 1.0520 1.0617 1.0789 1.1041 1.1561 1.2618 1.3614 1.5101
4.00 1.0000 1.0023 1.0046 1.0093 1.0186 1.0280 1.0375 1.0471 1.0544 1.0691 1.0914 1.1403 1.2303 1.3213 1.4555
Taken with permission from Ref. [9]. The value at the critical point (T r = P r = 1) is taken from the API-TDB [5]. Bold numbers indicate liquid region.
ig
Table 5.12, Z RA = 0.23. From Eq. (6.72), C = 127.7 J/mol · K, P 2 =−38.2557, and P 3 = 0.402287. From Table 6.1 for SRK
P
ig
2
T r = T/T c = 0.8, and P r = 0.198 = 0.2. From Tables 6.4 and EOS we have C P − C = 450 × 0.39565 − 450 × (3.887) /
∼
P
ig
ig
3
6.5, [(C P − C )/R] (0) = 3.564, [(C P − C )/R] (1) = 10.377. It is (−32.8406) − 83.14 = 301.936 cm · bar/mol · K. Since 1 J =
P P ig
3
important to note that the system is saturated liquid and 10 cm · bar, thus C P − C = 301.936/10 = 30.194 J/mol · K.
P
ig
ig
extrapolation of values for the liquid region from T r = 0.7 and C P = (C P − C ) + C = 30.194 + 127.7 = 157.9 J/mol · Kor
P
P
0.75–0.8 at P r = 0.2 is required for both (0) and (1) terms. C P = 157.9/78.1 = 2.02 J/g · C. The deviation with gener-
◦
Direct data given in the tables at T r = 0.8 and P r = 0.2 cor- alized correlation is –8.1%. Effect of considering volume
respond to saturated vapor and special care should be taken translation c on volume in calculation of C P is minor
when the system is at saturated conditions. From Eq. (6.57), and in this problem if V L directly calculated from SRK
ig
ig
3
[(C P − C )/R] = 5.74317, C P − C = 5.74317 × 8.314 = 47.8 equation (126.1 cm /mol) is used, value of calculated C P
P P
J/mol · K, and C P = 47.8 + 127.7 = 175.5 J/mol · K. The spe- would be still the same as 2.02 J/g · C. For calculation
◦
cific heat is calculated through molecular weight using of C V , SRK equation is used with relations given in Ta-
ig
3
Eq. (5.3) as C P = 175.5/78.1 = 2.25 J/g · C. This value is ble 6.1. C V − C = TP 3 = 450 × 0.402287 = 178.04 cm · bar/
◦
V
ig
ig
basically the same as the reported value. For SRK EOS, mol · K = 178.04/10 = 17.8 J/mol · K. C = C − R = 127.7 −
P
V
2
L
3
3
a = 1.907 × 10 7 bar(cm /mol) , b = 82.69 cm /mol, Z = 8.314 = 119.4 J/mol · K. Thus, C V = 119.4 + 17.8 = 137.2
3
3
L
0.033, V = 126.1cm /mol, c = 9.6cm /mol, and V = 116.4 J/mol · K = 137.2/78.1 = 1.75 J/g · .K. The heat capacity ratio
L
(corrected), which gives Z L (corrected) = 0.0305, where is γ = C P /C V = 2.02/1.75 = 1.151.
c is calculated from Eq. (5.51). From generalized corre- To calculate H − H ig from generalized correlations we
lations Z (0) = 0.0328 and Z (1) =−0.0138, which gives Z = get from Tables 6.2 and 6.3 as [(H − H )/RT c ] (0) =−4.518
ig
0.0299 that is very close to the value calculated from and [(H − H )/RT c ] (1) =−5.232. From Eq. (6.56), [(H − H )/
ig
ig
SRK EOS. Using units of kelvin for temperature, bar for RT c ] =−5.6167. Again it should be noted that the values of
3
3
pressure, and cm for volume, R = 83.14 cm · bar/mol · K [] (0) and [] (1) terms are taken for saturated liquid by extrap-
3
and V = 116.4cm /mol. From relations given in Table 6.1 olation of T r from 0.7 and 0.75 to 0.8. Values in the tables
we calculate a 1 =−33017 and a 2 = 60.9759. P 1 = 4.13169, for saturated vapor (at T r = 0.8, P r = 0.2) should be avoided.
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT