Page 340 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 340
QC: IML/FFX
T1: IML
P1: IML/FFX
P2: IML/FFX
AT029-Manual
17:40
AT029-Manual-v7.cls
June 22, 2007
AT029-07
320 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
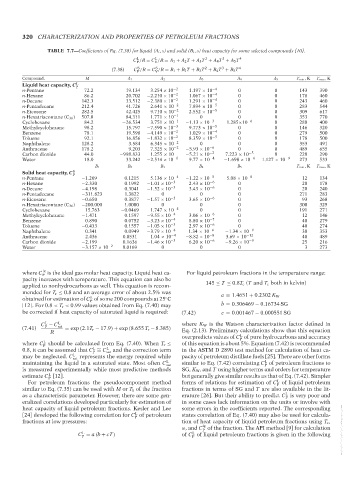
TABLE 7.7—Coefficients of Eq. (7.38) for liquid (A i , s) and solid (B i , s) heat capacity for some selected compounds [10].
L
2
3
L
C /R = C /R = A 1 + A 2 T + A 3 T + A 4 T + A 5 T 4
P V
S
2
3
S
(7.38) C /R = C /R = B 1 + B 2 T + B 3 T + B 4 T + B 5 T 4
P V
Compound. M A 1 A 2 A 3 A 4 A 5 T min ,K T max ,K
Liquid heat capacity, C L P
n-Pentane 72.2 19.134 −3.254 × 10 −2 1.197 × 10 −4 0 0 143 390
n-Hexane 86.2 20.702 −2.210 × 10 −2 1.067 × 10 −4 0 0 178 460
n-Decane 142.3 33.512 −2.380 × 10 −2 1.291 × 10 −4 0 0 243 460
n-Pentadecane 212.4 41.726 2.641 × 10 −2 7.894 × 10 −5 0 0 283 544
n-Eicosane 282.5 42.425 9.710 × 10 −2 2.552 × 10 −5 0 0 309 617
n-Hexatriacontane (C 36 ) 507.0 84.311 1.771 × 10 −1 0 0 0 353 770
Cyclohexane 84.2 −26.534 3.751 × 10 −1 −1.13 × 10 −3 1.285×10 −6 0 280 400
Methylcyclohexane 98.2 15.797 −7.590 × 10 −3 9.773 × 10 −5 0 0 146 320
Benzene 78.1 19.598 −4.149 × 10 −2 1.029 × 10 −4 0 0 279 500
Toluene 92.1 16.856 −1.832 × 10 −2 8.359 × 10 −5 0 0 178 500
Naphthalene 128.2 3.584 6.345 × 10 −2 0 0 0 353 491
Anthracene 178.2 9.203 7.325 × 10 −2 −5.93 × 10 −6 0 0 489 655
Carbon dioxide 44.0 −998.833 1.255 × 10 −5.21 × 10 −2 7.223 × 10 −5 0 220 290
Water 18.0 33.242 −2.514 × 10 −1 9.77 × 10 −4 −1.698 × 10 −6 1.127 × 10 −9 273 533
B 1 B 2 B 3 B 4 B 5 T min ,K T max ,K
Solid heat capacity, C S P
n-Pentane −1.209 0.1215 5.136 × 10 −4 −1.22 × 10 −5 5.08 × 10 −8 12 134
n-Hexane −2.330 0.1992 −1.01 × 10 −3 2.43 × 10 −6 0 20 178
n-Decane −4.198 0.3041 −1.52 × 10 −3 3.43 × 10 −6 0 20 240
n-Pentadecane −311.823 1.3822 0 0 0 271 283
n-Eicosane −0.650 0.3877 −1.57 × 10 −3 3.65 × 10 −6 0 93 268
n-Hexatriacontane (C 36 ) −200.000 1.0000 0 0 0 300 325
Cyclohexane 15.763 −0.0469 1.747 × 10 −4 0 0 191 271
Methylcyclohexane −1.471 0.1597 −9.55 × 10 −4 3.06 × 10 −6 0 12 146
Benzene 0.890 0.0752 −3.23 × 10 −4 8.80 × 10 −7 0 40 279
Toluene −0.433 0.1557 −1.05 × 10 −3 2.97 × 10 −6 0 40 274
Naphthalene 0.341 0.0949 −3.79 × 10 −4 1.34 × 10 −6 −1.34 × 10 −9 30 353
Anthracene 2.436 0.0531 1.04 × 10 −4 −8.82 × 10 −8 3.69 × 10 −12 40 489
Carbon dioxide −2.199 0.1636 −1.46 × 10 −3 6.20 × 10 −6 −9.26 × 10 −9 25 216
Water −3.157 × 10 −2 0.0169 0 0 0 3 273
ig
where C is the ideal gas molar heat capacity. Liquid heat ca- For liquid petroleum fractions in the temperature range:
P
pacity increases with temperature. This equation can also be
applied to nonhydrocarbons as well. This equation is recom- 145 ≤ T ≤ 0.8T c (T and T c both in kelvin)
mended for T r ≤ 0.8 and an average error of about 2.5% was
L
obtained for estimation of C of some 200 compounds at 25 C a = 1.4651 + 0.2302 K W
◦
P
[12]. For 0.8 < T r < 0.99 values obtained from Eq. (7.40) may b = 0.306469 − 0.16734 SG
be corrected if heat capacity of saturated liquid is required: (7.42) c = 0.001467 − 0.000551 SG
L
C − C L where K W is the Watson characterization factor defined in
(7.41) P sat = exp (2.1T r − 17.9) + exp (8.655 T r − 8.385)
R Eq. (2.13). Preliminary calculations show that this equation
L
overpredicts values of C of pure hydrocarbons and accuracy
P
L
where C should be calculated from Eq. (7.40). When T r ≤ of this equation is about 5%. Equation (7.42) is recommended
P
0.8, it can be assumed that C = C L sat and the correction term in the ASTM D 2890 test method for calculation of heat ca-
L ∼
P
may be neglected. C L sat represents the energy required while pacity of petroleum distillate fuels [25]. There are other forms
maintaining the liquid in a saturated state. Most often C sat similar to Eq. (7.42) correlating C of petroleum fractions to
L
L
P
is measured experimentally while most predictive methods SG, K W , and T using higher terms and orders for temperature
estimate C [12]. but generally give similar results as that of Eq. (7.42). Simpler
L
P
L
For petroleum fractions the pseudocomponent method forms of relations for estimation of C of liquid petroleum
P
similar to Eq. (7.35) can be used with M or T b of the fraction fractions in terms of SG and T are also available in the lit-
L
as a characteristic parameter. However, there are some gen- erature [26]. But their ability to predict C is very poor and
P
eralized correlations developed particularly for estimation of in some cases lack information on the units or involve with
heat capacity of liquid petroleum fractions. Kesler and Lee some errors in the coefficients reported. The corresponding
L
[24] developed the following correlation for C of petroleum states correlation of Eq. (7.40) may also be used for calcula-
P
fractions at low pressures: tion of heat capacity of liquid petroleum fractions using T c ,
ig
ω, and C of the fraction. The API method [9] for calculation --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
P
L
L
C = a (b + cT) of C of liquid petroleum fractions is given in the following
P P
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT