Page 1043 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1043
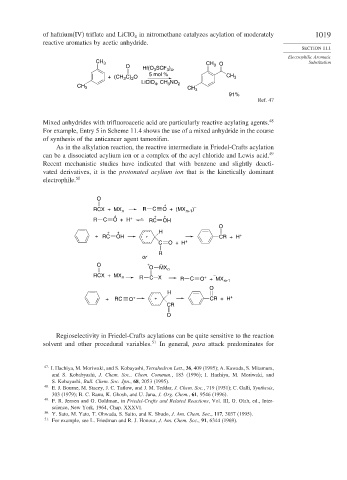
of hafnium(IV) triflate and LiClO in nitromethane catalyzes acylation of moderately 1019
4
reactive aromatics by acetic anhydride.
SECTION 11.1
Electrophilic Aromatic
CH 3 CH Substitution
O Hf(O SCF ) , 3 O
3 4
3
5 mol %
+ (CH C) O CH 3
3
2
LiClO 4 , CH NO 2
3
CH 3 CH 3
91%
Ref. 47
Mixed anhydrides with trifluoroacetic acid are particularly reactive acylating agents. 48
For example, Entry 5 in Scheme 11.4 shows the use of a mixed anhydride in the course
of synthesis of the anticancer agent tamoxifen.
As in the alkylation reaction, the reactive intermediate in Friedel-Crafts acylation
can be a dissociated acylium ion or a complex of the acyl chloride and Lewis acid. 49
Recent mechanistic studies have indicated that with benzene and slightly deacti-
vated derivatives, it is the protonated acylium ion that is the kinetically dominant
electrophile. 50
O
+
RCX + MX n R C O + (MX n+1 ) –
+ + +
R C O + H + RC OH
O
+ + H
+ RC OH + CR + H +
C O + H +
R
or
O + –
O MX n
RCX + MX n R C X R C O + MX n+1
–
+
O
H
+ RC O + + CR + H +
CR
O
Regioselectivity in Friedel-Crafts acylations can be quite sensitive to the reaction
solvent and other procedural variables. 51 In general, para attack predominates for
47 I. Hachiya, M. Moriwaki, and S. Kobayashi, Tetrahedron Lett., 36, 409 (1995); A. Kawada, S. Mitamura,
and S. Kobabyashi, J. Chem. Soc., Chem. Commun., 183 (1996); I. Hachiya, M. Moriwaki, and
S. Kobayashi, Bull. Chem. Soc. Jpn., 68, 2053 (1995).
48
E. J. Bourne, M. Stacey, J. C. Tatlow, and J. M. Teddar, J. Chem. Soc., 719 (1951); C. Galli, Synthesis,
303 (1979); B. C. Ranu, K. Ghosh, and U. Jana, J. Org. Chem., 61, 9546 (1996).
49 F. R. Jensen and G. Goldman, in Friedel-Crafts and Related Reactions, Vol. III, G. Olah, ed., Inter-
science, New York, 1964, Chap. XXXVI.
50 Y. Sato, M. Yato, T. Ohwada, S. Saito, and K. Shudo, J. Am. Chem. Soc., 117, 3037 (1995).
51
For example, see L. Friedman and R. J. Honour, J. Am. Chem. Soc., 91, 6344 (1969).