Page 1125 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1125
reactivity of the ketone toward nucleophilic addition and also makes the dioxirane 1101
intermediate more reactive.
SECTION 12.2
C H
12 25
Addition of Oxygen at
N + O Carbon-Carbon Double
Bonds
CH 3 O
PhCH CHCH OH
2
KOSO OOH PhCH CHCH OH
2
2
83%
The cyclic sulfone 4-thiopyrone-S S-dioxide also exhibits enhanced reactivity as a
result of the effect of the sulfone dipole. 103
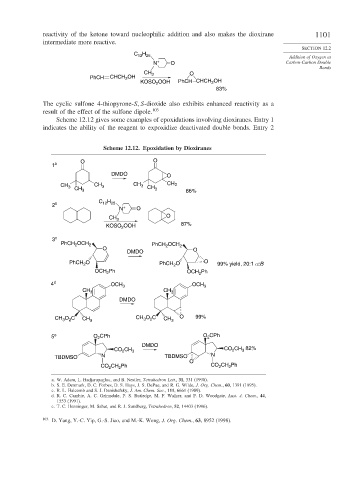
Scheme 12.12 gives some examples of epoxidations involving dioxiranes. Entry 1
indicates the ability of the reagent to expoxidize deactivated double bonds. Entry 2
Scheme 12.12. Epoxidation by Dioxiranes
O O
1 a
DMDO O
CH CH 3 CH 3 CH 3
3
CH 3 CH 3 86%
C H
12 25
2 b +
N O
CH 3 O
KOSO OOH 87%
2
3 c
PhCH OCH 2 PhCH OCH
2
O 2 2 O
DMDO
PhCH O PhCH O O 99% yield, 20:1 α:B
2
2
OCH Ph OCH Ph
2
2
4 d OCH 3 OCH 3
CH 3 CH 3
DMDO
CH O C CH 3 CH O C CH 3 O 99%
2
3
2
3
5 e O CPh O CPh
2
2
DMDO
CO CH 3 CO CH 82%
3
2
2
TBDMSO N TBDMSO N
O
CO CH Ph CO CH Ph
2
2
2
2
a. W. Adam, L. Hadjarapaglou, and B. Nestler, Tetrahedron Lett., 31, 331 (1990).
b. S. E. Denmark, D. C. Forbes, D. S. Hays, J. S. DePue, and R. G. Wilde, J. Org. Chem., 60, 1391 (1995).
c. R. L. Halcomb and S. J. Danishefsky, J. Am. Chem. Soc., 111, 6661 (1989).
d. R. C. Cambie, A. C. Grimsdale, P. S. Rutledge, M. F. Walker, and P. D. Woodgate, Aust. J. Chem., 44,
1553 (1991).
e. T. C. Henninger, M. Sabat, and R. J. Sundberg, Tetrahedron, 52, 14403 (1996).
103
D. Yang, Y.-C. Yip, G.-S. Jiao, and M.-K. Wong, J. Org. Chem., 63, 8952 (1998).