Page 317 - Advances in Biomechanics and Tissue Regeneration
P. 317
16.2 PROBLEM DESCRIPTION 315
main results obtained in such experiments. Next, we introduce the global mathematical framework, with the different
equations, parameters, and interactions between the corresponding functions and variables. The following section pre-
sents the implementation of such equations in a finite element (FE) framework. The weak forms of the differential equa-
tions as well as the matrix components of the algebraic system resulting from the FE approximation are derived. Also,
the corresponding results derived from the simulation results computed, after a process to identify the parameters that
best fit some of those results, are presented and discussed. Most of these examples correspond to problems that can be
assimilated to unidimensional, so the particularization of the global formulation to one dimension (1D) is used to get
such results. As a proof of concept, and to observe the full potential of the proposed approach, another example is also
solved and presented, now in 3D, although without experimental validation. Finally, the main conclusions of the work
are stated and commented upon.
16.2 PROBLEM DESCRIPTION
Taking into account the poor prognosis and complex structure of the GBM described above, it is clear that the devel-
opment of an accurate in vitro model for GBM research is very important. Three-dimensional cell cultures and micro-
fluidic systems can give us a lot of new and useful information, as they can reproduce much better the physiological
state and environment of a cell, which cannot be achieved in a standard 2D cell culture. As was said previously, the
main characteristics of GBM are the appearance of necrotic foci surrounded by areas of high cellularity (pseudopali-
sades) and microvascular proliferation. In our in vitro models, we will focus on the process of necrotic core and pseu-
dopalisade formation.
Uncontrolled proliferation of tumor cells and secretion of different factors induce occlusion of a surrounded blood
vessel. This causes a decrease in nutrient and oxygen supply, hence the appearance of hypoxia in the perivascular
region. Hypoxia provokes active cell migration away from this region and the formation of a hypercellular moving
wave (pseudopalisade). Tumor cells that do not migrate activate a process of apoptosis or necrosis, creating in that way
an enlarging necrotic zone. As the pseudopalisading cells are hypoxic, they have upregulated expression of the HIF,
which induces overexpression of the vascular endothelial growth factor (VEGF) that is responsible for microvascular
proliferation and hyperplasia. When the new blood vessels are formed, a pseudopalisade formation can be observed
around them. Once the cells reach a functional blood vessel with appropriate environmental conditions, they start to
proliferate at a high rate, and this can induce an occlusion of the vessel and restart the process [27].
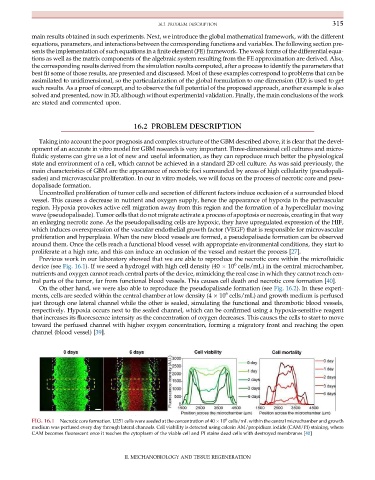
Previous work in our laboratory showed that we are able to reproduce the necrotic core within the microfluidic
6
device (see Fig. 16.1). If we seed a hydrogel with high cell density (40 10 cells/mL) in the central microchamber,
nutrients and oxygen cannot reach central parts of the device, mimicking the real case in which they cannot reach cen-
tral parts of the tumor, far from functional blood vessels. This causes cell death and necrotic core formation [40].
On the other hand, we were also able to reproduce the pseudopalisade formation (see Fig. 16.2). In these experi-
6
ments, cells are seeded within the central chamber at low density (4 10 cells/mL) and growth medium is perfused
just through one lateral channel while the other is sealed, simulating the functional and thrombotic blood vessels,
respectively. Hypoxia occurs next to the sealed channel, which can be confirmed using a hypoxia-sensitive reagent
that increases its fluorescence intensity as the concentration of oxygen decreases. This causes the cells to start to move
toward the perfused channel with higher oxygen concentration, forming a migratory front and reaching the open
channel (blood vessel) [39].
6
FIG. 16.1 Necrotic core formation. U251 cells were seeded at the concentration of 40 10 cells/mL within the central microchamber and growth
medium was perfused every day through lateral channels. Cell viability is detected using calcein AM/propidium iodide (CAM/PI) staining, where
CAM becomes fluorescent once it reaches the cytoplasm of the viable cell and PI stains dead cells with destroyed membranes [40]
II. MECHANOBIOLOGY AND TISSUE REGENERATION