Page 365 - Advances in Biomechanics and Tissue Regeneration
P. 365
362 18. CARTILAGE REGENERATION AND TISSUE ENGINEERING
in appositional cartilage growth). However, articular cartilage lacks a perichondrium, exhibiting, rather, smooth, lubri-
cated, synovial joint surfaces. Unlike most connective tissues, cartilage lacks vascularization; nutrients and metabolites
must reach cells via diffusion through the ECM. The avascular nature of cartilage is attributable to the fact that its
biochemical composition prevents vascular invasion; breakdown of the antiangiogenic cartilage barrier triggers
unwanted vascular invasion and irreversible cartilage degeneration [3].
There are three types of cartilage that differ in both appearance and mechanical properties and are characterized by
their matrices: hyaline cartilage, elastic cartilage, and fibrocartilage. Hyaline cartilage is the most abundant in humans;
this cartilage covers the articular surfaces of most synovial joints.
18.1.1 Cartilage Cells [1]
Cartilage cells are derived from mesenchymal stem cells, which are small undifferentiated cells with thin processes
exhibiting a high rate of proliferation; the cells can differentiate into chondroblasts and other types of cells. Chondro-
progenitor mesenchymal cells aggregate and differentiate into chondroblasts, which secrete the diverse components of
the cartilage matrix; when cells become completely surrounded by the matrix material that they have secreted, they are
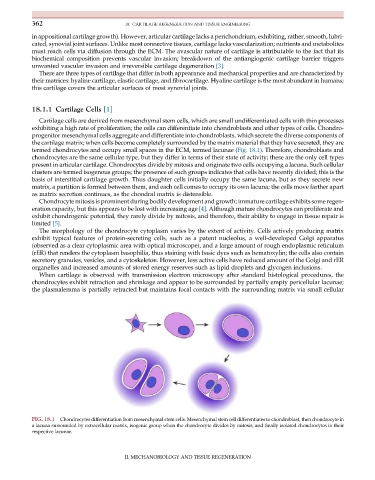
termed chondrocytes and occupy small spaces in the ECM, termed lacunae (Fig. 18.1). Therefore, chondroblasts and
chondrocytes are the same cellular type, but they differ in terms of their state of activity; these are the only cell types
present in articular cartilage. Chondrocytes divide by mitosis and originate two cells occupying a lacuna. Such cellular
clusters are termed isogenous groups; the presence of such groups indicates that cells have recently divided; this is the
basis of interstitial cartilage growth. Thus daughter cells initially occupy the same lacuna, but as they secrete new
matrix, a partition is formed between them, and each cell comes to occupy its own lacuna; the cells move farther apart
as matrix secretion continues, as the chondral matrix is distensible.
Chondrocyte mitosis is prominent during bodily development and growth; immature cartilage exhibits some regen-
eration capacity, but this appears to be lost with increasing age [4]. Although mature chondrocytes can proliferate and
exhibit chondrogenic potential, they rarely divide by mitosis, and therefore, their ability to engage in tissue repair is
limited [5].
The morphology of the chondrocyte cytoplasm varies by the extent of activity. Cells actively producing matrix
exhibit typical features of protein-secreting cells, such as a patent nucleolus, a well-developed Golgi apparatus
(observed as a clear cytoplasmic area with optical microscope), and a large amount of rough endoplasmic reticulum
(rER) that renders the cytoplasm basophilia, thus staining with basic dyes such as hematoxylin; the cells also contain
secretory granules, vesicles, and a cytoskeleton. However, less active cells have reduced amount of the Golgi and rER
organelles and increased amounts of stored energy reserves such as lipid droplets and glycogen inclusions.
When cartilage is observed with transmission electron microscopy after standard histological procedures, the
chondrocytes exhibit retraction and shrinkage and appear to be surrounded by partially empty pericellular lacunae;
the plasmalemma is partially retracted but maintains focal contacts with the surrounding matrix via small cellular
FIG. 18.1 Chondrocytes differentiation from mesenchymal stem cells. Mesenchymal stem cell differentiates to chondroblast, then chondrocyte in
a lacuna surrounded by extracellular matrix, isogenic group when the chondrocyte divides by mitosis, and finally isolated chondrocytes in their
respective lacunae.
II. MECHANOBIOLOGY AND TISSUE REGENERATION