Page 387 - Advances in Biomechanics and Tissue Regeneration
P. 387
384 19. IMPACT OF MECHANOBIOLOGICAL PERTURBATION IN CARTILAGE TISSUE ENGINEERING
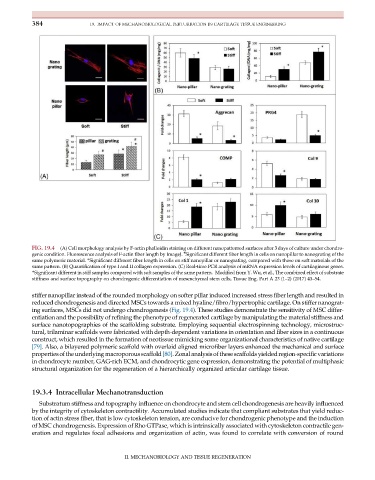
FIG. 19.4 (A) Cell morphology analysis by F-actin phalloidin staining on different nanopatterned surfaces after 3days of culture under chondro-
#
genic condition. Fluorescence analysis of F-actin fiber length by ImageJ. Significant different fiber length in cells on nanopillar to nanograting of the
same polymeric material. *Significant different fiber length in cells on stiff nanopillar or nanograting, compared with those on soft materials of the
same pattern. (B) Quantification of type I and II collagen expression. (C) Real-time PCR analysis of mRNA expression levels of cartilaginous genes.
*Significant different in stiff samples compared with soft samples of the same pattern. Modified from Y. Wu, et al., The combined effect of substrate
stiffness and surface topography on chondrogenic differentiation of mesenchymal stem cells, Tissue Eng. Part A 23 (1–2) (2017) 43–54.
stiffer nanopillar instead of the rounded morphology on softer pillar induced increased stress fiber length and resulted in
reduced chondrogenesis and directed MSCs towards a mixed hyaline/fibro/hypertrophic cartilage. On stiffer nanograt-
ing surfaces, MSCs did not undergo chondrogenesis (Fig. 19.4). These studies demonstrate the sensitivity of MSC differ-
entiation and the possibility of refining the phenotype of regenerated cartilage by manipulating the material stiffness and
surface nanotopographies of the scaffolding substrate. Employing sequential electrospinning technology, microstruc-
tural, trilaminar scaffolds were fabricated with depth-dependent variations in orientation and fiber sizes in a continuous
construct, which resulted in the formation of neotissue mimicking some organizational characteristics of native cartilage
[79]. Also, a bilayered polymeric scaffold with overlaid aligned microfiber layers enhanced the mechanical and surface
propertiesofthe underlying macroporousscaffold[80].Zonal analysis of these scaffolds yieldedregion-specific variations
in chondrocyte number, GAG-rich ECM, and chondrocytic gene expression, demonstrating the potential of multiphasic
structural organization for the regeneration of a hierarchically organized articular cartilage tissue.
19.3.4 Intracellular Mechanotransduction
Substratum stiffness and topography influence on chondrocyte and stem cell chondrogenesis are heavily influenced
by the integrity of cytoskeleton contractility. Accumulated studies indicate that compliant substrates that yield reduc-
tion of actin stress fiber, that is low cytoskeleton tension, are conducive for chondrogenic phenotype and the induction
of MSC chondrogenesis. Expression of Rho GTPase, which is intrinsically associated with cytoskeleton contractile gen-
eration and regulates focal adhesions and organization of actin, was found to correlate with conversion of round
II. MECHANOBIOLOGY AND TISSUE REGENERATION