Page 383 - Advances in Biomechanics and Tissue Regeneration
P. 383
380 19. IMPACT OF MECHANOBIOLOGICAL PERTURBATION IN CARTILAGE TISSUE ENGINEERING
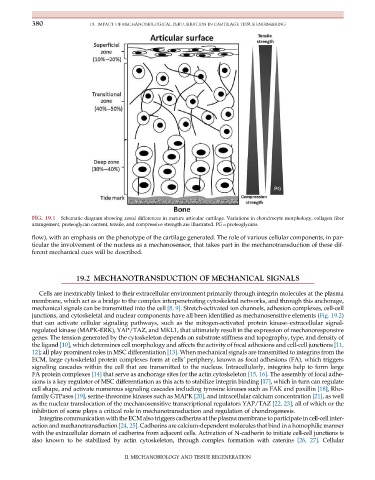
FIG. 19.1 Schematic diagram showing zonal differences in mature articular cartilage. Variations in chondrocyte morphology, collagen fiber
arrangement, proteoglycan content, tensile, and compressive strength are illustrated. PG¼proteoglycans.
flow), with an emphasis on the phenotype of the cartilage generated. The role of various cellular components, in par-
ticular the involvement of the nucleus as a mechanosensor, that takes part in the mechanotransduction of these dif-
ferent mechanical cues will be described.
19.2 MECHANOTRANSDUCTION OF MECHANICAL SIGNALS
Cells are inextricably linked to their extracellular environment primarily through integrin molecules at the plasma
membrane, which act as a bridge to the complex interpenetrating cytoskeletal networks, and through this anchorage,
mechanical signals can be transmitted into the cell [8, 9]. Stretch-activated ion channels, adhesion complexes, cell-cell
junctions, and cytoskeletal and nuclear components have all been identified as mechanosensitive elements (Fig. 19.2)
that can activate cellular signaling pathways, such as the mitogen-activated protein kinase–extracellular signal-
regulated kinase (MAPK-ERK), YAP/TAZ, and MKL1, that ultimately result in the expression of mechanoresponsive
genes. The tension generated by the cytoskeleton depends on substrate stiffness and topography, type, and density of
the ligand [10], which determines cell morphology and affects the activity of focal adhesions and cell-cell junctions [11,
12]; all play prominent roles in MSC differentiation [13]. When mechanical signals are transmitted to integrins from the
ECM, large cytoskeletal protein complexes form at cells’ periphery, known as focal adhesions (FA), which triggers
signaling cascades within the cell that are transmitted to the nucleus. Intracellularly, integrins help to form large
FA protein complexes [14] that serve as anchorage sites for the actin cytoskeleton [15, 16]. The assembly of focal adhe-
sions is a key regulator of MSC differentiation as this acts to stabilize integrin binding [17], which in turn can regulate
cell shape, and activate numerous signaling cascades including tyrosine kinases such as FAK and paxillin [18], Rho-
family GTPases [19], serine-threonine kinases such as MAPK [20], and intracellular calcium concentration [21], as well
as the nuclear translocation of the mechanosensitive transcriptional regulators YAP/TAZ [22, 23], all of which or the
inhibition of some plays a critical role in mechanotransduction and regulation of chondrogenesis.
Integrins communication with the ECM also triggers cadherins at the plasma membrane to participate in cell-cell inter-
action and mechanotransduction [24, 25]. Cadherins are calcium-dependent molecules that bind in a homophilic manner
with the extracellular domain of cadherins from adjacent cells. Activation of N-cadherin to initiate cell-cell junctions is
also known to be stabilized by actin cytoskeleton, through complex formation with catenins [26, 27].Cellular
II. MECHANOBIOLOGY AND TISSUE REGENERATION