Page 384 - Advances in Biomechanics and Tissue Regeneration
P. 384
19.3 INFLUENCE OF EXTRACELLULAR CUES 381
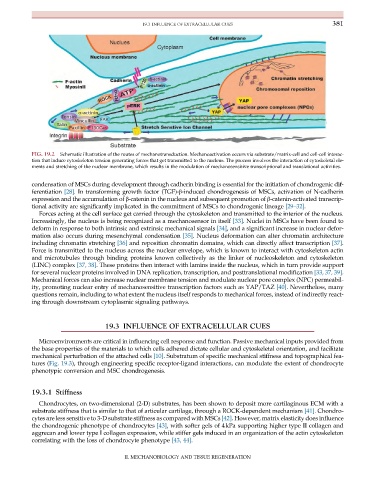
FIG. 19.2 Schematic illustration of the routes of mechanotransduction. Mechanoactivation occurs via substrate/matrix-cell and cell-cell interac-
tion that induce cytoskeleton tension generating forces that get transmitted to the nucleus. The process involves the interaction of cytoskeletal ele-
ments and stretching of the nuclear membrane, which results in the modulation of mechanosensitive transcriptional and translational activities.
condensation of MSCs during development through cadherin binding is essential for the initiation of chondrogenic dif-
ferentiation [28]. In transforming growth factor (TGF)-β-induced chondrogenesis of MSCs, activation of N-cadherin
expression and the accumulation of β-catenin in the nucleus and subsequent promotion of β-catenin-activated transcrip-
tional activity are significantly implicated in the commitment of MSCs to chondrogenic lineage [29–32].
Forces acting at the cell surface get carried through the cytoskeleton and transmitted to the interior of the nucleus.
Increasingly, the nucleus is being recognized as a mechanosensor in itself [33]. Nuclei in MSCs have been found to
deform in response to both intrinsic and extrinsic mechanical signals [34], and a significant increase in nuclear defor-
mation also occurs during mesenchymal condensation [35]. Nucleus deformation can alter chromatin architecture
including chromatin stretching [36] and reposition chromatin domains, which can directly affect transcription [37].
Force is transmitted to the nucleus across the nuclear envelope, which is known to interact with cytoskeleton actin
and microtubules through binding proteins known collectively as the linker of nucleoskeleton and cytoskeleton
(LINC) complex [37, 38]. These proteins then interact with lamins inside the nucleus, which in turn provide support
for several nuclear proteins involved in DNA replication, transcription, and posttranslational modification [33, 37, 39].
Mechanical forces can also increase nuclear membrane tension and modulate nuclear pore complex (NPC) permeabil-
ity, promoting nuclear entry of mechanosensitive transcription factors such as YAP/TAZ [40]. Nevertheless, many
questions remain, including to what extent the nucleus itself responds to mechanical forces, instead of indirectly react-
ing through downstream cytoplasmic signaling pathways.
19.3 INFLUENCE OF EXTRACELLULAR CUES
Microenvironments are critical in influencing cell response and function. Passive mechanical inputs provided from
the base properties of the materials to which cells adhered dictate cellular and cytoskeletal orientation, and facilitate
mechanical perturbation of the attached cells [10]. Substratum of specific mechanical stiffness and topographical fea-
tures (Fig. 19.3), through engineering specific receptor-ligand interactions, can modulate the extent of chondrocyte
phenotypic conversion and MSC chondrogenesis.
19.3.1 Stiffness
Chondrocytes, on two-dimensional (2-D) substrates, has been shown to deposit more cartilaginous ECM with a
substrate stiffness that is similar to that of articular cartilage, through a ROCK-dependent mechanism [41]. Chondro-
cytes are less sensitive to 3-D substrate stiffness as compared with MSCs [42]. However, matrix elasticity does influence
the chondrogenic phenotype of chondrocytes [43], with softer gels of 4kPa supporting higher type II collagen and
aggrecan and lower type I collagen expression, while stiffer gels induced in an organization of the actin cytoskeleton
correlating with the loss of chondrocyte phenotype [43, 44].
II. MECHANOBIOLOGY AND TISSUE REGENERATION