Page 387 - Analysis, Synthesis and Design of Chemical Processes, Third Edition
P. 387
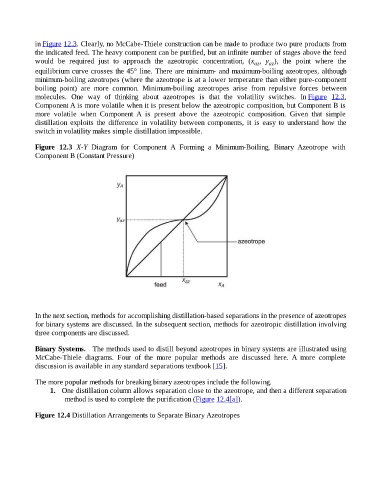
in Figure 12.3. Clearly, no McCabe-Thiele construction can be made to produce two pure products from
the indicated feed. The heavy component can be purified, but an infinite number of stages above the feed
would be required just to approach the azeotropic concentration, (x , y ), the point where the
az az
equilibrium curve crosses the 45° line. There are minimum- and maximum-boiling azeotropes, although
minimum-boiling azeotropes (where the azeotrope is at a lower temperature than either pure-component
boiling point) are more common. Minimum-boiling azeotropes arise from repulsive forces between
molecules. One way of thinking about azeotropes is that the volatility switches. In Figure 12.3,
Component A is more volatile when it is present below the azeotropic composition, but Component B is
more volatile when Component A is present above the azeotropic composition. Given that simple
distillation exploits the difference in volatility between components, it is easy to understand how the
switch in volatility makes simple distillation impossible.
Figure 12.3 X-Y Diagram for Component A Forming a Minimum-Boiling, Binary Azeotrope with
Component B (Constant Pressure)
In the next section, methods for accomplishing distillation-based separations in the presence of azeotropes
for binary systems are discussed. In the subsequent section, methods for azeotropic distillation involving
three components are discussed.
Binary Systems. The methods used to distill beyond azeotropes in binary systems are illustrated using
McCabe-Thiele diagrams. Four of the more popular methods are discussed here. A more complete
discussion is available in any standard separations textbook [15].
The more popular methods for breaking binary azeotropes include the following.
1. One distillation column allows separation close to the azeotrope, and then a different separation
method is used to complete the purification (Figure 12.4[a]).
Figure 12.4 Distillation Arrangements to Separate Binary Azeotropes