Page 389 - Analysis, Synthesis and Design of Chemical Processes, Third Edition
P. 389
An example of this situation is the ethanol-water system, which has a binary azeotrope in the 90–95 mol%
ethanol range (depending on system pressure). A relatively recent method for purifying ethanol beyond the
azeotropic composition is pervaporation [16]. The following question may arise when considering this
method: Why not just use pervaporation (or whatever second separation method is possible) for the entire
separation? The answer is that, even with volatile energy prices, the relatively low cost of energy makes
distillation a very economical separation method [16]. In most cases, an arrangement like Figure 12.4(a)
is far less expensive than using the second separation method alone. This is because separations such as
distillation are very economical for producing relatively pure products from roughly equal mixtures.
Obtaining ultrapure products from distillation can have unfavorable economics, because large numbers of
trays are required for very high purity (think about the McCabe-Thiele construction). Separations like
pervaporation (or any membrane separation) have much more favorable economics when removing a
dilute component from a relatively pure component. They are also economically unattractive for large
processing volumes. Therefore, the combination of distillation and another separation like pervaporation
usually provides the economic optimum.
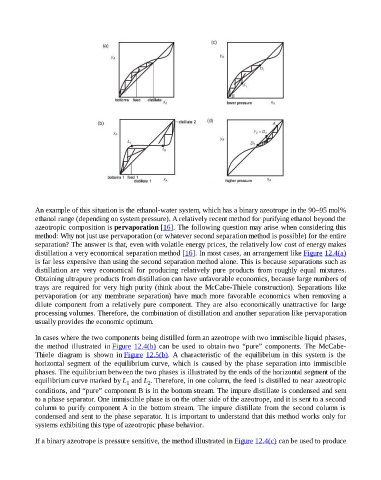
In cases where the two components being distilled form an azeotrope with two immiscible liquid phases,
the method illustrated in Figure 12.4(b) can be used to obtain two “pure” components. The McCabe-
Thiele diagram is shown in Figure 12.5(b). A characteristic of the equilibrium in this system is the
horizontal segment of the equilibrium curve, which is caused by the phase separation into immiscible
phases. The equilibrium between the two phases is illustrated by the ends of the horizontal segment of the
equilibrium curve marked by L and L . Therefore, in one column, the feed is distilled to near azeotropic
1 2
conditions, and “pure” component B is in the bottom stream. The impure distillate is condensed and sent
to a phase separator. One immiscible phase is on the other side of the azeotrope, and it is sent to a second
column to purify component A in the bottom stream. The impure distillate from the second column is
condensed and sent to the phase separator. It is important to understand that this method works only for
systems exhibiting this type of azeotropic phase behavior.
If a binary azeotrope is pressure sensitive, the method illustrated in Figure 12.4(c) can be used to produce